Bistable Functions and Signaling Motifs in Systems Chemistry: Taking the Next Step Toward Synthetic Cells
- PMID: 39841921
- PMCID: PMC11800382
- DOI: 10.1021/acs.accounts.4c00703
Bistable Functions and Signaling Motifs in Systems Chemistry: Taking the Next Step Toward Synthetic Cells
Abstract
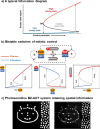
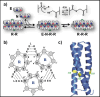
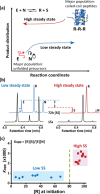
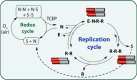
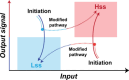
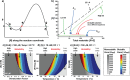
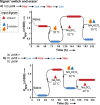
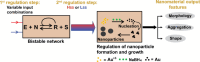
A key challenge in modern chemistry research is to mimic life-like functions using simple molecular networks and the integration of such networks into the first functional artificial cell. Central to this endeavor is the development of signaling elements that can regulate the cell function in time and space by producing entities of code with specific information to induce downstream activity. Such artificial signaling motifs can emerge in nonequilibrium systems, exhibiting complex dynamic behavior like bistability, multistability, oscillations, and chaos. However, the de novo, bottom-up design of such systems remains challenging, primarily because the kinetic characteristics and energy aspects yielding bifurcation have not yet been globally defined. We herein review our recent work that focuses on the design and functional analysis of peptide-based networks, propelled by replication reactions and exhibiting bistable behavior. Furthermore, we rationalize and discuss their exploitation and implementation as variable signaling motifs in homogeneous and heterogeneous environments.The bistable reactions constitute reversible second-order autocatalysis as positive feedback to generate two distinct product distributions at steady state (SS), the low-SS and high-SS. Quantitative analyses reveal that a phase transition from simple reversible equilibration dynamics into bistability takes place when the system is continuously fueled, using a reducing agent, to keep it far from equilibrium. In addition, an extensive set of experimental, theoretical, and simulation studies highlight a defined parameter space where bistability operates.Analogous to the arrangement of protein-based bistable motifs in intracellular signaling pathways, sequential concatenation of the synthetic bistable networks is used for signal processing in homogeneous media. The cascaded network output signals are switched and erased or transduced by manipulating the order of addition of the components, allowing it to reach "on demand" either the low-SS or high-SS. The pre-encoded bistable networks are also useful as a programming tool for the downstream regulation of nanoscale materials properties, bridging together the Systems Chemistry and Nanotechnology fields. In such heterogeneous cascade pathways, the outputs of the bistable network serve as input signals for consecutive nanoparticle formation reaction and growth processes, which-depending on the applied conditions-regulate various features of (Au) nanoparticle shape and assembly.Our work enables the design and production of various signaling apparatus that feature higher complexity than previously observed in chemical networks. Future studies, briefly discussed at the end of the Account, will be directed at the design and analysis of more elaborate functionality, such as bistability under flow conditions, multistability, and oscillations. We propose that a profound understanding of the design principles facilitating the replication-based bistability and related functions bear implications for exploring the origin of protein functionality prior to the highly evolved replication-translation-transcription machinery. The integration of our peptide-based signaling motifs within future synthetic cells seems to be a straightforward development of the two alternating states as memory and switch elements for controlling cell growth and division and even communication among different cells. We furthermore suggest that such systems can be introduced into living cells for various biotechnology applications, such as switches for cell temporal and spatial manipulations.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
A chemically fueled non-enzymatic bistable network.Nat Commun. 2019 Oct 11;10(1):4636. doi: 10.1038/s41467-019-12645-0. Nat Commun. 2019. PMID: 31604941 Free PMC article.
-
Signaling in Systems Chemistry: Programing Gold Nanoparticles Formation and Assembly Using a Dynamic Bistable Network.Angew Chem Int Ed Engl. 2021 Feb 23;60(9):4512-4517. doi: 10.1002/anie.202012837. Epub 2020 Nov 10. Angew Chem Int Ed Engl. 2021. PMID: 33006406 Free PMC article.
-
Emergent Bistable Switches from the Incoherent Feed-Forward Signaling of a Positive Feedback Loop.ACS Synth Biol. 2021 Nov 19;10(11):3117-3128. doi: 10.1021/acssynbio.1c00373. Epub 2021 Oct 25. ACS Synth Biol. 2021. PMID: 34694110
-
Artificial Cells: Synthetic Compartments with Life-like Functionality and Adaptivity.Acc Chem Res. 2017 Apr 18;50(4):769-777. doi: 10.1021/acs.accounts.6b00512. Epub 2017 Jan 17. Acc Chem Res. 2017. PMID: 28094501 Free PMC article. Review.
-
Grip on complexity in chemical reaction networks.Beilstein J Org Chem. 2017 Jul 28;13:1486-1497. doi: 10.3762/bjoc.13.147. eCollection 2017. Beilstein J Org Chem. 2017. PMID: 28845192 Free PMC article. Review.
References
-
- Maity I.; Dev D.; Cohen-Luria R.; Wagner N.; Ashkenasy G. Engineering reaction networks by sequential signal processing. Chem. 2024, 10, 1132–1146. 10.1016/j.chempr.2023.10.017. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

