Neoadjuvant Chemotherapy for Newly Diagnosed, Advanced Ovarian Cancer: ASCO Guideline Update
- PMID: 39841949
- PMCID: PMC11934100
- DOI: 10.1200/JCO-24-02589
Neoadjuvant Chemotherapy for Newly Diagnosed, Advanced Ovarian Cancer: ASCO Guideline Update
Abstract
Purpose: To provide updated guidance regarding neoadjuvant chemotherapy (NACT) and primary cytoreductive surgery (PCS) among patients with stage III-IV epithelial ovarian, fallopian tube, or primary peritoneal cancer (epithelial ovarian cancer [EOC]).
Methods: A multidisciplinary Expert Panel convened and updated the systematic review.
Results: Sixty-one studies form the evidence base.
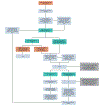
Recommendations: Patients with suspected stage III-IV EOC should be evaluated by a gynecologic oncologist, with cancer antigen 125, computed tomography of the abdomen and pelvis, and chest imaging included. All patients with EOC should be offered germline genetic and somatic testing at diagnosis. For patients with newly diagnosed advanced EOC who are fit for surgery and have a high likelihood of achieving complete cytoreduction, PCS is recommended. For patients fit for PCS but deemed unlikely to have complete cytoreduction, NACT is recommended. Patients with newly diagnosed advanced EOC and a high perioperative risk profile should receive NACT. Before NACT, patients should have histologic confirmation of invasive ovarian cancer. For NACT, a platinum-taxane doublet is recommended. Interval cytoreductive surgery (ICS) should be performed after ≤four cycles of NACT for patients with a response to chemotherapy or stable disease. For patients with stage III disease, good performance status, and adequate renal function treated with NACT, hyperthermic intraperitoneal chemotherapy may be offered during ICS. After ICS, chemotherapy should continue to complete a six-cycle treatment plan with the optional addition of bevacizumab. Patients with EOC should be offered US Food and Drug Administration-approved maintenance treatments. Patients with progressive disease on NACT should have diagnosis reconfirmed via tissue biopsy. Patients without previous comprehensive genetic or molecular profiling should be offered testing. Treatment options include alternative chemotherapy regimens, clinical trials, and/or initiation of end-of-life care.Additional information is available at www.asco.org/gynecologic-cancer-guidelines.This guideline has been endorsed by the Society of Gynecologic Oncology.
Conflict of interest statement
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at DOI
Neoadjuvant Chemotherapy for Newly Diagnosed, Advanced Ovarian Cancer: ASCO Guideline Update
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I 5 Immediate Family Member, Inst 5 My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Stéphanie Gaillard
Deborah K. Armstrong
Mitchell I. Edelson
Agustin A. Garcia
Gregory M. Gressel
Jamie L. Lesnock
Larissa A. Meyer
Kathleen N. Moore
Roisin E. O’Cearbhaill
Aptitude Health
Alexander B. Olawaiye
Ritu Salani
No other potential conflicts of interest were reported.
Figures

References
-
- Bristow RE, Tomacruz RS, Armstrong DK, et al. : Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: A meta-analysis. J Clin Oncol 41:4065–4076, 2023 - PubMed
-
- Vergote I, Tropé CG, Amant F, et al. : Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med 363:943–953, 2010 - PubMed
-
- Kehoe S, Hook J, Nankivell M, et al. : Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): An open-label, randomised, controlled, non-inferiority trial. Lancet 386:249–257, 2015 - PubMed
-
- Fagotti A, Ferrandina G, Vizzielli G, et al. : Phase III randomised clinical trial comparing primary surgery versus neoadjuvant chemotherapy in advanced epithelial ovarian cancer with high tumour load (SCORPION trial): Final analysis of peri-operative outcome. Eur J Cancer 59:22–33, 2016 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

