Pediatric relapsed/refractory ALK-positive anaplastic large cell lymphoma treatment and outcomes in the targeted-drug era
- PMID: 39841960
- PMCID: PMC11954107
- DOI: 10.1182/bloodadvances.2024014745
Pediatric relapsed/refractory ALK-positive anaplastic large cell lymphoma treatment and outcomes in the targeted-drug era
Abstract
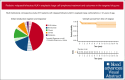
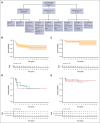
Treatment options for patients with relapsed or refractory (R/R) anaplastic large cell lymphoma (ALCL) have increased in the era of targeted therapies such as brentuximab vedotin (BV) and anaplastic lymphoma kinase (ALK) inhibitors. However, there is no standard treatment and published data evaluating their use are limited. The goal of this retrospective study was to describe current real-world treatment and outcomes of pediatric, adolescent, and young adult patients with R/R ALK-positive ALCL. We conducted a retrospective, multi-institutional study identifying 81 patients with R/R ALK-positive ALCL aged ≤21 years at initial diagnosis treated between 2011 and 2022 across 18 institutions. Median time from diagnosis to relapse was 8.9 months (range, 2.6-131.9). Initial reinduction regimens included ALK-inhibitor monotherapy (n = 37, 46%), BV monotherapy (n = 19, 23%), chemotherapy without targeted therapy (n = 12, 15%), chemotherapy with targeted therapy (n = 9, 11%), or vinblastine monotherapy (n = 4, 5%), with 83% of patients achieving a complete response to initial reinduction regimen. Fifty-eight patients received a hematopoietic stem cell transplant (HSCT), 11 autologous and 48 allogeneic, with 1 receiving both. Duration of treatment for patients receiving BV or the ALK-inhibitor crizotinib (CZ) varied widely (BV, 1-11 years; CZ, 2-10 years). Five-year event-free survival was 63% (95% confidence interval [CI], 53-75) and 5-year overall survival was 91% (95% CI, 84-98). This is, to our knowledge, the largest collection of patients with R/R ALK-positive ALCL treated in the era of targeted therapy. Patients achieved excellent responses to ALK-inhibitor or BV monotherapy, but questions remain about duration of therapy and role of HSCT.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures

References
-
- Sandlund JT, Downing JR, Crist WM. Non-Hodgkin's lymphoma in childhood. N Engl J Med. 1996;334(19):1238–1248. - PubMed
-
- Alexander S, Kraveka JM, Weitzman S, et al. Advanced stage anaplastic large cell lymphoma in children and adolescents: results of ANHL0131, a randomized phase III trial of APO versus a modified regimen with vinblastine: a report from the Children's Oncology Group. Pediatr Blood Cancer. 2014;61(12):2236–2242. - PMC - PubMed
-
- Le Deley MC, Rosolen A, Williams DM, et al. Vinblastine in children and adolescents with high-risk anaplastic large-cell lymphoma: results of the randomized ALCL99-vinblastine trial. J Clin Oncol. 2010;28(25):3987–3993. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

