Effectiveness of a Web-Based Medication Education Course on Pregnant Women's Medication Information Literacy and Decision Self-Efficacy: Randomized Controlled Trial
- PMID: 39841986
- PMCID: PMC11799814
- DOI: 10.2196/54148
Effectiveness of a Web-Based Medication Education Course on Pregnant Women's Medication Information Literacy and Decision Self-Efficacy: Randomized Controlled Trial
Abstract
Background: Medication-related adverse events are common in pregnant women, and most are due to misunderstanding medication information. The identification of appropriate medication information sources requires adequate medical information literacy (MIL). It is important for pregnant women to comprehensively evaluate the risk of medication treatment, self-monitor their medication response, and actively participate in decision-making to reduce medication-related adverse events.
Objective: This study aims to examine the effectiveness of a medication education course on a web-based platform in improving pregnant women's MIL and decision self-efficacy.
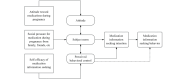
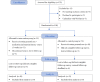
Methods: A randomized controlled trial was conducted. Pregnant women were recruited from January to June 2021 in the Department of Obstetrics and Gynecology of a large hospital in a major city in central China. A total of 108 participants were randomly divided into a control group (CG), which received routine prenatal care from nurses and physicians, and an intervention group (IG), which received an additional 3-week web-based medication education course based on the theory of planned behavior as part of routine prenatal care. Participants completed a Medication Information Literacy Scale and a decision self-efficacy questionnaire at baseline, upon completion of the intervention, and at a 4-week follow-up. Generalized estimation equations (GEE) were used to analyze the main effect (time and grouping) and interaction effect (grouping×time) of the 2 outcomes. The CONSORT-EHEALTH (V 1.6.1) checklist was used to guide the reporting of this randomized controlled trial.
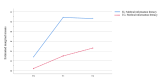
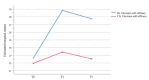
Results: A total of 91 pregnant women (48 in the IG and 43 in the CG) completed the questionnaires at the 3 time points. The results of GEE indicated that there was no statistically significant difference in time×group interactions of MIL between the 2 groups (F2=3.12; P=.21). The results of the main effect analysis showed that there were statistically significant differences in MIL between the 2 groups at T1 and T2 (F1=17.79; P<.001). Moreover, the results of GEE indicated that there was a significant difference in decision self-efficacy regarding the time factor, grouping factor, and time×group interactions (F2=21.98; P<.001). The results of the simple effect analysis indicated a statistically significant difference in decision self-efficacy between the 2 groups at T1 (F1=36.29; P<.001) and T2 (F1=36.27; P<.001) compared to T0. Results showed that MIL and decision self-efficacy in the IG were found to be significantly higher than those in the CG (d=0.81; P<.001 and d=1.26; P<.001, respectively), and they remained significantly improved at the 4-week follow-up (d=0.59; P<.001 and d=1.27; P<.001, respectively).
Conclusions: Web-based medication education courses based on the theory of planned behavior can effectively improve pregnant women's MIL and decision self-efficacy, and they can be used as supplementary education during routine prenatal care.
Trial registration: Chinese Clinical Trial Registry ChiCTR2100041817; https://www.chictr.org.cn/showproj.html?proj=66685.
Keywords: RCT; decision efficacy; decision self-efficacy; information literacy; medication education; medication information literacy; pregnancy; pregnant women; randomized controlled trial; self-efficacy; web-based medication education; web-based platforms.
©Suya Li, Hui-Jun Chen, Jie Zhou, Yi-Bei Zhouchen, Rong Wang, Jinyi Guo, Sharon R Redding, Yan-Qiong Ouyang. Originally published in the Journal of Medical Internet Research (https://www.jmir.org), 22.01.2025.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures
Similar articles
-
Sertindole for schizophrenia.Cochrane Database Syst Rev. 2005 Jul 20;2005(3):CD001715. doi: 10.1002/14651858.CD001715.pub2. Cochrane Database Syst Rev. 2005. PMID: 16034864 Free PMC article.
-
Adefovir dipivoxil and pegylated interferon alfa-2a for the treatment of chronic hepatitis B: a systematic review and economic evaluation.Health Technol Assess. 2006 Aug;10(28):iii-iv, xi-xiv, 1-183. doi: 10.3310/hta10280. Health Technol Assess. 2006. PMID: 16904047
-
Home treatment for mental health problems: a systematic review.Health Technol Assess. 2001;5(15):1-139. doi: 10.3310/hta5150. Health Technol Assess. 2001. PMID: 11532236
-
Shared decision-making for people with asthma.Cochrane Database Syst Rev. 2017 Oct 3;10(10):CD012330. doi: 10.1002/14651858.CD012330.pub2. Cochrane Database Syst Rev. 2017. PMID: 28972652 Free PMC article.
-
Psychosocial interventions for supporting women to stop smoking in pregnancy.Cochrane Database Syst Rev. 2017 Feb 14;2(2):CD001055. doi: 10.1002/14651858.CD001055.pub5. Cochrane Database Syst Rev. 2017. PMID: 28196405 Free PMC article.
Cited by
-
Protein Counting as an Educational Strategy to Optimize Low-Protein-Diet Adherence and Satisfaction in Stage 4 and 5 Chronic Kidney Disease Patients: A Pilot Study.Nutrients. 2025 Apr 25;17(9):1438. doi: 10.3390/nu17091438. Nutrients. 2025. PMID: 40362747 Free PMC article.
References
-
- Ke AB, Greupink R, Abduljalil K. Drug dosing in pregnant women: challenges and opportunities in using physiologically based pharmacokinetic modeling and simulations. CPT Pharmacometrics Syst Pharmacol. 2018;7(2):103–110. doi: 10.1002/psp4.12274. https://europepmc.org/abstract/MED/29349870 - DOI - PMC - PubMed
-
- Bedewi N, Sisay M, Edessa D. Drug utilization pattern among pregnant women attending maternal and child health clinic of tertiary hospital in eastern Ethiopia: consideration of toxicological perspectives. BMC Res Notes. 2018;11(1):858. doi: 10.1186/s13104-018-3966-5. https://bmcresnotes.biomedcentral.com/articles/10.1186/s13104-018-3966-5 10.1186/s13104-018-3966-5 - DOI - DOI - PMC - PubMed
-
- Thorpe PG, Gilboa SM, Hernandez-Diaz S, Lind J, Cragan JD, Briggs G, Kweder S, Friedman JM, Mitchell AA, Honein MA. Medications in the first trimester of pregnancy: most common exposures and critical gaps in understanding fetal risk. Pharmacoepidemiol Drug Saf. 2013;22(9):1013–1018. doi: 10.1002/pds.3495. https://europepmc.org/abstract/MED/23893932 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

