Time to start taking time seriously: how to investigate unexpected biological rhythms within infectious disease research
- PMID: 39842489
- PMCID: PMC11753885
- DOI: 10.1098/rstb.2023.0336
Time to start taking time seriously: how to investigate unexpected biological rhythms within infectious disease research
Abstract
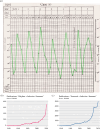
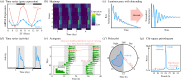
The discovery of rhythmicity in host and pathogen activities dates back to the Hippocratic era, but the causes and consequences of these biological rhythms have remained poorly understood. Rhythms in infection phenotypes or traits are observed across taxonomically diverse hosts and pathogens, suggesting general evolutionary principles. Understanding these principles may enable rhythms to be leveraged in manners that improve drug and vaccine efficacy or disrupt pathogen timekeeping to reduce virulence and transmission. Explaining and exploiting rhythms in infections require an integrative and multidisciplinary approach, which is a hallmark of research within chronobiology. Many researchers are welcomed into chronobiology from other fields after observing an unexpected rhythm or time-of-day effect in their data. Such findings can launch a rich new research topic, but engaging with the concepts, approaches and dogma in a new discipline can be daunting. Fortunately, chronobiology has well-developed frameworks for interrogating rhythms that can be readily applied in novel contexts. Here, we provide a 'how to' guide for exploring unexpected daily rhythms in infectious disease research. We outline how to establish: whether the rhythm is circadian, to what extent the host and pathogen are responsible, the relevance for host-pathogen interactions, and how to explore therapeutic potential.This article is part of the Theo Murphy meeting issue 'Circadian rhythms in infection and immunity'.
Keywords: circadian clock; fitness; host–parasite interaction; immune response; rhythms; seasonal.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Wright HK. 1901. The malarial fevers of british malaya. Singapore, China: Kelly & Walsh. See https://archive.org/details/b29000841/page/n3/mode/2up.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
