Breast milk delivery of an engineered dimeric IgA protects neonates against rotavirus
- PMID: 39842610
- PMCID: PMC11982437
- DOI: 10.1016/j.mucimm.2025.01.002
Breast milk delivery of an engineered dimeric IgA protects neonates against rotavirus
Abstract
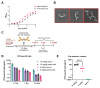
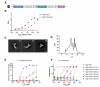
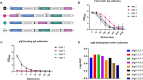
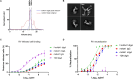
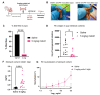
Dimeric IgA (dIgA) is the dominant antibody in many mucosal tissues. It is actively transported onto mucosal surfaces as secretory IgA (sIgA) which plays an integral role in protection against enteric pathogens, particularly in young children. Therapeutic strategies that deliver engineered, potently neutralizing antibodies directly into the infant intestine through breast milk could provide enhanced antimicrobial protection for neonates. Here, we developed a murine model of maternal protective transfer against human rotavirus (RV) using systemic administration of a dimeric IgA monoclonal antibody (mAb). First, we showed that systemically administered dIgA passively transferred into breast milk and the stomach of suckling pups in a dose-dependent manner. Next, we optimized the recombinant production of a potently RV-neutralizing, VP4-specific dIgA (mAb41) antibody. We then demonstrated that systemic administration of dIgA and IgG mAb41 in lactating dams conferred protection from RV-induced diarrhea in suckling pups, with dIgA resulting in lower diarrhea incidence from IgG. Systemic delivery of engineered antimicrobial dIgA mAbs should be considered as an effective strategy for sIgA delivery to the infant gastrointestinal tract via breast milk to increase protection against enteric pathogens.
Keywords: Breast milk; Dimeric IgA; Maternal immunity; Neonatal immunity; Passive transfer; Rotavirus.
Copyright © 2025 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: [J.E.C. has served as a consultant for Luna Biologics, is a member of the Scientific Advisory Board of Meissa Vaccines and is Founder of IDBiologics. The Crowe laboratory at Vanderbilt University Medical Center has received unrelated sponsored research agreements from Takeda Vaccines, IDBiologics and AstraZeneca. S.R.P. provides individual consulting services to Moderna, Merck, Dynavax, GSK, and Pfizer on CMV vaccines. Merck Vaccines and Moderna have provided grants and contracts for S.R.P. sponsored programs.].
Figures

References
-
- Chodirker W.B., Tomasi T.B., Jr. Gamma-globulins: quantitative relationships in human serum and nonvascular fluids. Science. 1963;142(3595):1080–1081. - PubMed
-
- Renegar K.B., Small P.A., Jr. Passive transfer of local immunity to influenza virus infection by IgA antibody. J Immunol. 1991;146(6):1972–1978. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

