The clinical and molecular landscape of diffuse hemispheric glioma, H3 G34-mutant
- PMID: 39842935
- PMCID: PMC12309718
- DOI: 10.1093/neuonc/noaf015
The clinical and molecular landscape of diffuse hemispheric glioma, H3 G34-mutant
Abstract
Background: Diffuse hemispheric glioma, histone 3 (H3) G34-mutant, has been newly defined in the 2021 World Health Organization (WHO) classification of central nervous system tumors. Here we sought to define the prognostic roles of clinical, neuroimaging, pathological, and molecular features of these tumors.
Methods: We retrospectively assembled a cohort of 114 patients (median age 22 years) with diffuse hemispheric glioma, H3 G34-mutant, central nervous system WHO grade 4, and profiled the imaging, histological, and molecular landscape of their tumors.
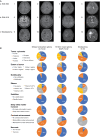
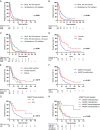
Results: Compared with glioblastoma, H3 G34-mutant diffuse hemispheric gliomas exhibited less avid contrast enhancement, necrosis, and edema on MRI. Comprehensive analyses of mutational and DNA copy number profiles revealed recurrent mutations in TP53 and ATRX, homozygous deletions of CDKN2A/B, and amplifications of PDGFRA, EGFR, CCND2, and MYCN. MGMT promoter methylation was detected in 79 tumors (75%); 11 tumors (13%) showed DNA copy number profiles suggestive of circumscribed deletions on 10q26.3 involving the MGMT locus. Median survival was 21.5 months. Female sex, gross total resection, and MGMT promoter methylation were positive prognostic factors on univariate analysis. Among radiological, pathological, and molecular features, the absence of pial invasion and the presence of microvascular proliferation and CDK6 amplification were positive prognostic factors on univariate analyses.
Conclusions: This study refines the clinical and molecular landscape of H3 G34-mutant diffuse hemispheric gliomas. Dedicated trials for this novel tumor type are urgently needed.
Keywords: MGMT; glioblastoma; histone; loss; methylation.
© The Author(s) 2025. Published by Oxford University Press on behalf of the Society for Neuro-Oncology.
Conflict of interest statement
E.L.R. has received research grants from Bristol Meyers Squibb (BMS), and honoraria for lectures or advisory board participation or consulting from Astra Zeneca Daiichi, Bayer, Biodexa/Sitoxi, Janssen, Leo Pharma, Pfizer, Pierre Fabre, Roche, Seattle Genetics, and Servier.
A.B. declares no conflicts of interest.
J.F. declares no conflicts of interest.
D.G. declares no conflicts of interest.
S.B. has received honoraria for lectures or advisory board participation from Bayer and B. Braun.
J.K.B. declares no conflicts of interest.
A.W. declares no conflicts of interest.
J.C.T. has received honoraria for consulting AAA-Novartis and for traveling costs from Servier.
M.M. declares no conflict of interest.
G.T. has served on advisory boards (Bayer, Boehringer Ingelheim, CureVac, Miltenyi Biomedicine, Novocure), as a consultant (Bayer, Boehringer Ingelheim, CureVac), as steering committee member in noninterventional trials (Bayer, Novocure), as a speaker (Novocure, Servier), and financial compensation for all these activities was provided as institutional funding to the University Hospital Tübingen.
D.C. has received research grants from Novocure and is a cofunder and shareholder of Heidelberg Epignostix GmbH.
M.S. is a scientific Advisor, and Shareholder for HAloDx, Heidelberg Epignostix, and a scientific advisor for InnoSIGN and Arima Genomics.
E.R. has received honoraria and conference attendance support by Astra Zeneca, Servier, Gilead, Pfizer, Novartis, and Glaxo.
M.W.R. reports a research grant from UCB as well as an honoraria for advisory board participation from Alexion.
N.N. declares no conflict of interest.
H.-K.N. declares no conflicts of interest.
U.P. declares no conflict of interest.
T.B. declares no conflicts of interest.
S.Q. declares no conflicts of interest.
D.R. declares no conflicts of interest.
U.S. declares no conflicts of interest.
J.O. received research grants from Novocure and Olympus.
K.D. declares no conflicts of interest.
C.-A.M. declares no conflicts of interest.
L.R. declares no conflicts of interest.
E.H. declares no conflicts of interest.
M.G. declares no conflicts of interest.
T.H. declares no conflicts of interest.
S.P. declares no conflicts of interest.
L.B. declares no conflicts of interest.
T.L. declares no conflicts of interest.
V.M. declares no conflicts of interest.
P.S. declares no conflicts of interest.
D.S. declares no conflicts of interest.
W.W. declares no conflicts of interest.
F.S. is a cofounder and shareholder of Heidelberg Epignostix GmbH.
G.R. declares no conflicts of interest.
A.v.D. declares no conflicts of interest.
M.W. has received research grants from Novartis, Quercis, and Versameb, and honoraria for lectures or advisory board participation or consulting from Bayer, CureVac, Medac, Novartis, Novocure, Orbus, Philogen, Roche, Sandoz and Servier.
Figures


References
-
- Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016; 131(6):803–820. - PubMed
-
- Sturm D, Witt H, Hovestadt V, et al. Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer Cell. 2012; 22(4):425–437. - PubMed
-
- Schwartzentruber J, Korshunov A, Liu XY, et al. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature. 2012; 482(7384):226–231. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

