Myosin-based nucleation of actin filaments contributes to stereocilia development critical for hearing
- PMID: 39843411
- PMCID: PMC11754657
- DOI: 10.1038/s41467-025-55898-8
Myosin-based nucleation of actin filaments contributes to stereocilia development critical for hearing
Abstract
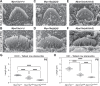
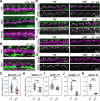
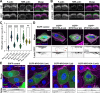
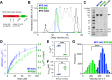
Assembly of actin-based stereocilia is critical for cochlear hair cells to detect sound. To tune their mechanosensivity, stereocilia form bundles composed of graded rows of ascending height, necessitating the precise control of actin polymerization. Myosin 15 (MYO15A) drives hair bundle development by delivering critical proteins to growing stereocilia that regulate actin polymerization via an unknown mechanism. Here, we show that MYO15A is itself an actin nucleation-promoting factor. Moreover, a deafness-causing mutation in the MYO15A actin-binding interface inhibits nucleation activity but still preserves some movement on filaments in vitro and partial trafficking on stereocilia in vivo. Stereocilia fail to elongate correctly in this mutant mouse, providing evidence that MYO15A-driven actin nucleation contributes to hair bundle biogenesis. Our work shows that in addition to generating force and motility, the ATPase domain of MYO15A can directly regulate actin polymerization and that disrupting this activity can promote cytoskeletal disease, such as hearing loss.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures


References
-
- Tilney, L. G., Cotanche, D. A. & Tilney, M. S. Actin filaments, stereocilia and hair cells of the bird cochlea. VI. How the number and arrangement of stereocilia are determined. Dev. Camb. Engl.116, 213–226 (1992). - PubMed
MeSH terms
Substances
Grants and funding
- F31 DC021881/DC/NIDCD NIH HHS/United States
- MC_U142684175/RCUK | Medical Research Council (MRC)
- F31DC021881/U.S. Department of Health & Human Services | NIH | National Institute on Deafness and Other Communication Disorders (NIDCD)
- Z01 DC000039/ImNIH/Intramural NIH HHS/United States
- DC000039/U.S. Department of Health & Human Services | NIH | National Institute on Deafness and Other Communication Disorders (NIDCD)
- HL006049/U.S. Department of Health & Human Services | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- T32 DC000039/DC/NIDCD NIH HHS/United States
- MR/X004597/1/RCUK | Medical Research Council (MRC)
- MC_UP_1503/2/RCUK | Medical Research Council (MRC)
- R01DC018827/U.S. Department of Health & Human Services | NIH | National Institute on Deafness and Other Communication Disorders (NIDCD)
- R01 DC018827/DC/NIDCD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

