Lactic acid in the vaginal milieu modulates the Candida-host interaction
- PMID: 39843417
- PMCID: PMC11760238
- DOI: 10.1080/21505594.2025.2451165
Lactic acid in the vaginal milieu modulates the Candida-host interaction
Abstract
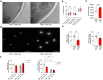
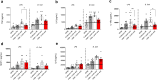
Vulvovaginal candidiasis (VVC) is one of the most common infections caused by Candida albicans. VVC is characterized by an inadequate hyperinflammatory response and clinical symptoms associated with Candida colonization of the vaginal mucosa. Compared to other host niches in which C. albicans can cause infection, the vaginal environment is extremely rich in lactic acid that is produced by the vaginal microbiota. We examined how lactic acid abundance in the vaginal niche impacts the interaction between C. albicans and the human immune system using an in vitro culture in vaginal simulative medium (VSM). The presence of lactic acid in VSM (VSM+LA) increased C. albicans proliferation, hyphal length, and its ability to cause damage during subsequent infection of vaginal epithelial cells. The cell wall of C. albicans cells grown in VSM+LA displayed a robust mannan fibrillar structure, β-glucan exposure, and low chitin content. These cell wall changes were associated with altered immune responses and an increased ability of the fungus to induce trained immunity. Neutrophils were compromised in clearing C. albicans grown in VSM+LA conditions, despite mounting stronger oxidative responses. Collectively, we found that fungal adaptation to lactic acid in a vaginal simulative context increases its immunogenicity favouring a pro-inflammatory state. This potentially contributes to the immune response dysregulation and neutrophil recruitment observed during recurrent VVC.
Keywords: Vulvovaginal candidiasis; candida albicans; host response; lactic acid; vaginal simulative medium.
Conflict of interest statement
M.G.N. and Leo A.B. Joosten are scientific co-founders of TTxD, and Lemba. M.G.N. is a scientific co-founder of BioTRIP. The other authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
