Enantioselective Heck/Tsuji-Trost reaction of flexible vinylic halides with 1,3-dienes
- PMID: 39843426
- PMCID: PMC11754474
- DOI: 10.1038/s41467-025-56142-z
Enantioselective Heck/Tsuji-Trost reaction of flexible vinylic halides with 1,3-dienes
Abstract
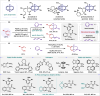
The enantioselective domino Heck/cross-coupling has emerged as a powerful tool in modern chemical synthesis for decades. Despite significant progress in relative rigid skeleton substrates, the implementation of asymmetric Heck/cross-coupling cascades of highly flexible haloalkene substrates remains a challenging and and long-standing goal. Here we report an efficient asymmetric domino Heck/Tsuji-Trost reaction of highly flexible vinylic halides with 1,3-dienes enabled by palladium catalysis. Specifically, the Heck insertion as stereodetermining step to form ƞ3 allyl palladium complex and in situ trapping with nucleophiles enable efficient Heck/etherification in a formal (4 + 2) cycloaddition manner. Engineering the Sadphos bearing androgynous non-C2-symmetric chiral sulfinamide phosphine ligands are vital component in achieving excellent catalytic reactivity and enantioselectivity. This strategy offers a general, modular and divergent platform for rapidly upgrading feedstock flexible vinylic halides and dienes to various value-added molecules and is expected to inspire the development of other challenging enantioselective domino Heck/cross-couplings.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




References
-
- Pellissier, H. Recent developments in enantioselective metal-catalyzed domino reactions. Adv. Synth. Catal.361, 1733–1755 (2019). - DOI
-
- Tietze, L.-F. Domino Reactions: Concepts for Efficient Organic Synthesis. (Wiley-VCH, Weinheim, Germany, 2014).
-
- Tietze, L.-F., Brasche, G., Gericke, K. M. Domino Reactions in Organic Synthesis. (Wiley-VCH, Weinheim, Germany, 2006).
Grants and funding
LinkOut - more resources
Full Text Sources

