Promoting in-situ stability of hydroxide exchange membranes by thermally conductive network for durable water electrolysis
- PMID: 39843436
- PMCID: PMC11754833
- DOI: 10.1038/s41467-025-56262-6
Promoting in-situ stability of hydroxide exchange membranes by thermally conductive network for durable water electrolysis
Abstract
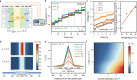
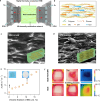
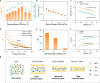
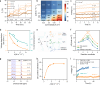
Hydroxide exchange membrane (HEM) water electrolysis is promising for green hydrogen production due to its low cost and excellent performance. However, HEM often has insufficient stability in strong alkaline solutions, particularly under in-situ electrolysis operation conditions, hindering its commercialization. In this study, we discover that the in-situ stability of HEM is primarily impaired by the locally accumulated heat in HEM due to its low thermal conductivity. Accordingly, we propose highly thermally conductive HEMs with an efficient three-dimensional (3D) thermal diffusion network to promote the in-situ stability of HEM for water electrolysis. Based on the 3D heat conductive network, the thermal conductivity of polymeric HEM is boosted by 32 times and thereby reduce the HEM temperature by up to 4.9 °C in a water electrolyzer at the current density of 1 A cm-2. Thus, the thermally conductive HEM exhibits negligible degradation after 20,000 start/stop cycles and reduces the degradation rate by 6 times compared to the pure polymeric HEM in a water electrolyzer. This study manifests the significance of thermal conductivity of HEM on the durability of water electrolysis, which provides guidelines on the rational design of highly durable HEMs in practical operation conditions for water electrolysis, fuel cells, and beyond.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

References
-
- Brandt, J. et al. Cost and competitiveness of green hydrogen and the effects of the European Union regulatory framework. Nat. Energy9, 703–713 (2024). - DOI
-
- Hu, X. et al. An operationally broadened alkaline water electrolyser enabled by highly stable poly(oxindole biphenylene) ion-solvating membranes. Nat. Energy9, 401–410 (2024). - DOI
-
- Luo, X. et al. Porous PVA skin-covered thin Zirfon-type separator as a new approach boosting high-rate alkaline water electrolysis beyond 1000 hours’ lifespan. eScience4, 100290 (2024).
LinkOut - more resources
Full Text Sources

