The molecular basis of Human FN3K mediated phosphorylation of glycated substrates
- PMID: 39843453
- PMCID: PMC11754801
- DOI: 10.1038/s41467-025-56207-z
The molecular basis of Human FN3K mediated phosphorylation of glycated substrates
Abstract
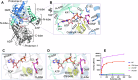
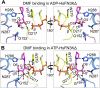
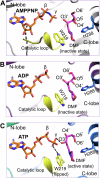
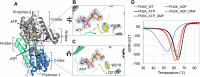
Glycation, a non-enzymatic post-translational modification occurring on proteins, can be actively reversed via site-specific phosphorylation of the fructose-lysine moiety by FN3K kinase, to impact the cellular function of the target protein. A regulatory axis between FN3K and glycated protein targets has been associated with conditions like diabetes and cancer. However, the molecular basis of this relationship has not been explored so far. Here, we determined a series of crystal structures of HsFN3K in the apo-state, and in complex with different nucleotide analogs together with a sugar substrate mimic to reveal the features important for its kinase activity and substrate recognition. Additionally, the dynamics in sugar substrate binding during the kinase catalytic cycle provide important mechanistic insights into HsFN3K function. Our structural work provides the molecular basis for rational small molecule design targeting FN3K.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



Update of
-
The molecular basis of Human FN3K mediated phosphorylation of glycated substrate.bioRxiv [Preprint]. 2024 Aug 5:2024.08.05.606604. doi: 10.1101/2024.08.05.606604. bioRxiv. 2024. Update in: Nat Commun. 2025 Jan 22;16(1):941. doi: 10.1038/s41467-025-56207-z. PMID: 39149269 Free PMC article. Updated. Preprint.
References
-
- Hodge, J. E. The Amadori rearrangement. Adv Carbohydr Chem10, 169–205 (1955). - PubMed
-
- Baynes, J. W. et al. The Amadori product on protein: structure and reactions. Prog Clin Biol Res304, 43–67 (1989). - PubMed
-
- Brownlee, M. Glycosylation products as toxic mediators of diabetic complications. Annu Rev Med42, 159–166 (1991). - PubMed
-
- Ahmed, M. U., Thorpe, S. R. & Baynes, J. W. Identification of N epsilon-carboxymethyllysine as a degradation product of fructoselysine in glycated protein. J Biol Chem261, 4889–4894 (1986). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

