Microbial carbon oxidation in seawater below the hypoxic threshold
- PMID: 39843462
- PMCID: PMC11754627
- DOI: 10.1038/s41598-024-82438-z
Microbial carbon oxidation in seawater below the hypoxic threshold
Abstract
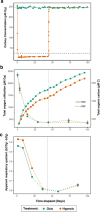
Global oxygen minimum zones (OMZs) often reach hypoxia but seldom reach anoxia. Recently it was reported that Michaelis Menten constants (Km) of oxidative enzymes are orders of magnitude higher than respiratory Km values, and in the Hypoxic Barrier Hypothesis it was proposed that, in ecosystems experiencing falling oxygen, oxygenase enzyme activities become oxygen-limited long before respiration. We conducted a mesocosm experiment with a phytoplankton bloom as an organic carbon source and controlled dissolved oxygen (DO) concentrations in the dark to determine whether hypoxia slows carbon oxidation and oxygen decline. Total oxygen utilization (TOU) in hypoxic treatment (ca. 7.1 µM O2) was 21.7% lower than the oxic treatment (ca. 245.1 µM O2) over the first 43 days of the experiment. In addition, following the restoration of fully oxic conditions to the hypoxic treatment, TOU accelerated, demonstrating that oxidative processes are sensitive to DO concentrations found in large volumes of the ocean. Microbial amplicon-based community composition diverged between oxic treatments, indicating a specialized microbiome that included Thioglobaceae (SUP05 Gammaproteobacteria), OM190 (Planctomycetota), ABY1 (Patescibacteria), and SAR86 subclade D2472, thrived in the hypoxic treatment, while the genus Candidatus Actinomarina and SAR11 alphaproteobacteria were sharply inhibited. Our findings support the hypothesis that oxygenase kinetics might slow the progression of ocean deoxygenation in oxygen-poor regions and be a factor in the evolution of microbial taxa adapted to hypoxic environments.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures



Similar articles
-
Biochemical Barriers on the Path to Ocean Anoxia?mBio. 2021 Aug 31;12(4):e0133221. doi: 10.1128/mBio.01332-21. Epub 2021 Jul 13. mBio. 2021. PMID: 34253057 Free PMC article.
-
Microbial Activities and Selection from Surface Ocean to Subseafloor on the Namibian Continental Shelf.Appl Environ Microbiol. 2022 May 10;88(9):e0021622. doi: 10.1128/aem.00216-22. Epub 2022 Apr 11. Appl Environ Microbiol. 2022. PMID: 35404072 Free PMC article.
-
The Physiology and Biogeochemistry of SUP05.Ann Rev Mar Sci. 2022 Jan 3;14:261-275. doi: 10.1146/annurev-marine-010419-010814. Epub 2021 Aug 20. Ann Rev Mar Sci. 2022. PMID: 34416125
-
Fish Ecology and Evolution in the World's Oxygen Minimum Zones and Implications of Ocean Deoxygenation.Adv Mar Biol. 2016;74:117-98. doi: 10.1016/bs.amb.2016.04.001. Epub 2016 Jun 17. Adv Mar Biol. 2016. PMID: 27573051 Review.
-
Microbial oceanography of anoxic oxygen minimum zones.Proc Natl Acad Sci U S A. 2012 Oct 2;109(40):15996-6003. doi: 10.1073/pnas.1205009109. Epub 2012 Sep 11. Proc Natl Acad Sci U S A. 2012. PMID: 22967509 Free PMC article. Review.
References
-
- Oschlies, A., Brandt, P., Stramma, L. & Schmidtko, S. Drivers and mechanisms of ocean deoxygenation. Nat. Geosci.11, 467–473. 10.1038/s41561-018-0152-2 (2018).
-
- Keeling, R. E., Kortzinger, A. & Gruber, N. Ocean deoxygenation in a warming world. Ann. Rev. Mar. Sci.2, 199–229. 10.1146/annurev.marine.010908.163855 (2010). - PubMed
-
- Schmidtko, S., Stramma, L. & Visbeck, M. Decline in global oceanic oxygen content during the past five decades. Nature542, 335–339 (2017). - PubMed
-
- Howarth, R. W. Nutrient limitation of net primary production in marine ecosystems. Ann. Rev. Ecol. Syst.19, 89–110 (1988).
-
- Duce, R. A. et al. Impacts of atmospheric anthropogenic nitrogen on the open ocean. Science320, 893–897 (2008). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

