Saponin-based adjuvant uptake and induction of antigen cross-presentation by CD11b+ dendritic cells and macrophages
- PMID: 39843492
- PMCID: PMC11754886
- DOI: 10.1038/s41541-024-01056-y
Saponin-based adjuvant uptake and induction of antigen cross-presentation by CD11b+ dendritic cells and macrophages
Abstract
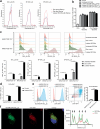
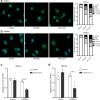
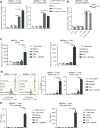
Saponin-based adjuvants (SBAs) distinguish themselves as vaccine adjuvants by instigating a potent activation of CD8+ T cells. Previously, we discovered SBA's ability to induce cross-presentation in dendritic cells (DCs) leading to CD8+ T cell activation. Moreover, the MHCIIloCD11bhi bone marrow-derived DC (BMDC) subset was identified to be the most responsive DC subset to SBA treatment. To further investigate SBA's mode of action, labeling of SBAs was optimized with the fluorescent dye SP-DiIC18(3). Efficient uptake of SBAs occurs specifically by MHCIIloCD11bhi BMDCs and bone marrow-derived macrophages (BMDMs) in vitro and cDC2s and macrophages ex vivo. Furthermore, SBAs are primarily taken up by clathrin-mediated endocytosis and uptake induces lipid bodies and antigen translocation to the cytosol in MHCIIloCD11bhi BMDCs and BMDMs. Importantly, BMDMs treated with SBAs exhibit cross-presentation leading to potent CD8+ T cells activation. Our findings explain the potency of SBAs as vaccine adjuvants and contribute to vaccine development.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures


Similar articles
-
Saponin-based adjuvant-induced dendritic cell cross-presentation is dependent on PERK activation.Cell Mol Life Sci. 2022 Apr 9;79(5):231. doi: 10.1007/s00018-022-04253-x. Cell Mol Life Sci. 2022. PMID: 35396971 Free PMC article.
-
Saponin-based adjuvants enhance antigen cross-presentation in human CD11c+ CD1c+ CD5- CD163+ conventional type 2 dendritic cells.J Immunother Cancer. 2023 Aug;11(8):e007082. doi: 10.1136/jitc-2023-007082. J Immunother Cancer. 2023. PMID: 37612044 Free PMC article.
-
Saponin-based adjuvants induce cross-presentation in dendritic cells by intracellular lipid body formation.Nat Commun. 2016 Nov 7;7:13324. doi: 10.1038/ncomms13324. Nat Commun. 2016. PMID: 27819292 Free PMC article.
-
Adjuvants Enhancing Cross-Presentation by Dendritic Cells: The Key to More Effective Vaccines?Front Immunol. 2018 Dec 13;9:2874. doi: 10.3389/fimmu.2018.02874. eCollection 2018. Front Immunol. 2018. PMID: 30619259 Free PMC article. Review.
-
Salmonella infection of bone marrow-derived macrophages and dendritic cells: influence on antigen presentation and initiating an immune response.FEMS Immunol Med Microbiol. 2000 Apr;27(4):313-20. doi: 10.1111/j.1574-695X.2000.tb01445.x. FEMS Immunol Med Microbiol. 2000. PMID: 10727887 Review.
Cited by
-
Eliciting antitumor immunity via therapeutic cancer vaccines.Cell Mol Immunol. 2025 Aug;22(8):840-868. doi: 10.1038/s41423-025-01316-4. Epub 2025 Jul 9. Cell Mol Immunol. 2025. PMID: 40629076 Free PMC article. Review.
-
Matrix-M adjuvant triggers inflammasome activation and enables antigen cross-presentation through induction of lysosomal membrane permeabilization.NPJ Vaccines. 2025 Aug 5;10(1):184. doi: 10.1038/s41541-025-01243-5. NPJ Vaccines. 2025. PMID: 40764493 Free PMC article.
-
Harnessing Thalassochemicals: Marine Saponins as Bioactive Agents in Nutraceuticals and Food Technologies.Mar Drugs. 2025 May 26;23(6):227. doi: 10.3390/md23060227. Mar Drugs. 2025. PMID: 40559636 Free PMC article. Review.
References
-
- den Brok, M. H. et al. Saponin-based adjuvants create a highly effective anti-tumor vaccine when combined with in situ tumor destruction. Vaccine30, 737–744 (2012). - PubMed
-
- Kensil, C. R., Patel, U., Lennick, M. & Marciani, D. Separation and characterization of saponins with adjuvant activity from Quillaja saponaria Molina cortex. J. Immunol.146, 431–437 (1991). - PubMed
-
- Morein, B., Sundquist, B., Höglund, S., Dalsgaard, K. & Osterhaus, A. Iscom, a novel structure for antigenic presentation of membrane proteins from enveloped viruses. Nature308, 457–460 (1984). - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

