Stalled ribosome rescue factors exert different roles depending on types of antibiotics in Escherichia coli
- PMID: 39843510
- PMCID: PMC11721466
- DOI: 10.1038/s44259-024-00039-2
Stalled ribosome rescue factors exert different roles depending on types of antibiotics in Escherichia coli
Abstract
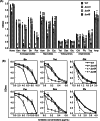
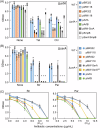
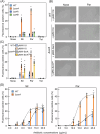
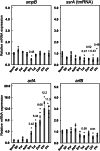
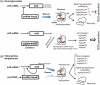
Escherichia coli possesses three stalled-ribosome rescue factors, tmRNA·SmpB (primary factor), ArfA (alternative factor to tmRNA·SmpB), and ArfB. Here, we examined the susceptibility of rescue factor-deficient strains from E. coli SE15 to various ribosome-targeting antibiotics. Aminoglycosides specifically decreased the growth of the ΔssrA (tmRNA gene) strain, in which the levels of reactive oxygen species were elevated. The decrease in growth of ΔssrA could not be complemented by plasmid-borne expression of arfA, arfB, or ssrAAA to DD mutant gene possessing a proteolysis-resistant tag sequence. These results highlight the significance of tmRNA·SmpB-mediated proteolysis during growth under aminoglycoside stress. In contrast, tetracyclines or amphenicols decreased the growth of the ΔarfA strain despite the presence of tmRNA·SmpB. Quantitative RT-PCR revealed that tetracyclines and amphenicols, but not aminoglycosides, considerably induced mRNA expression of arfA. These findings indicate that tmRNA·SmpB, and ArfA exert differing functions during stalled-ribosome rescue depending on the type of ribosome-targeting antibiotic.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

References
LinkOut - more resources
Full Text Sources

