Spectroscopic investigation of two xanthane dyes and design of a FRET based pesticide sensor
- PMID: 39843511
- PMCID: PMC11754873
- DOI: 10.1038/s41598-024-84846-7
Spectroscopic investigation of two xanthane dyes and design of a FRET based pesticide sensor
Abstract
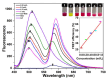
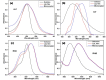
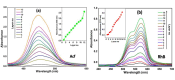
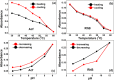
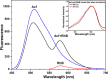
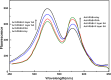
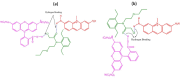
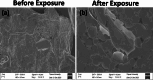
Layer-by-Layer (LbL) technique is the simplest and inexpensive method for preparartion of nano-dimensional thin films for tailoring material behavior having wide range of applications including sensors. Here, spectroscopic behavior of two laser dyes Acriflavine (Acf) and Rhodamine B (RhB) assembled onto LbL films have been investigated. It has been observed that both Acf and RhB form stable LbL films. Polyanion polyacrylic acid (PAA) was used to incorporate the Acf or RhB onto the LbL films. Adsorption of Acf and RhB onto PAA were completed within 45 min and 30 min respectively. During LbL film, material loss occurred in case of Acf. It has been demonstrated that such material loss can be minimized by incorporating clay laponite onto the LbL films. Temperature and pH dependant studies indicate that Acf and RhB assembled onto LbL films can be used to design temperature as well as pH sensors. Fluorescence Resonance Energy Transfer (FRET) between Acf and RhB has also been investigated. Interestingly, it has been demonstrated that the energy transfer efficiency can be manipulated using spacer molecules within the Acf and RhB LbL films. Laponite clay can be used to enhance the FRET efficiency, whereas stearic acid (SA) can be used to lower the efficiency. FRET efficiency linearly changes upon exposure of a pesticide pretilachlor at varying concentration. This study indicated that with proper calibration, proposed sensing system can be used to design FRET based pesticide sensor with detection limit of 0.22 ppm.
Keywords: Clay; Dyes; FRET; PAA; Pretilachlor; SA.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures



References
-
- Nix, W. D. Mechanical properties of thin films. Metall. Trans. A. 20, 2217–2245 (1989). - DOI
-
- d’Auria, L., Huignard, J. P., Roy, A. M. & Spitz, E. Photolithographic fabrication of thin film lenses. Opt. Commun.5, 232–235 (1972). - DOI
-
- Handbook of Laser Technology and Applications. (Three- Volume Set) 1675 (2003).
-
- Hussain, S. et al. Tuning of polydiacetylene phase behavior mixed with cholesterol derivative and its application toward the detection of pathogenic bacteria. J. Mater. Sci.58, 15762–15779 (2023). - DOI
-
- Rahman, F. Y. et al. Resistive switching behavior employing the Ipomoea carnea Plant for Biodegradable Rewritable read-only memory applications. ACS Appl. Electron. Mater.5, 3685–3697 (2023). - DOI
Grants and funding
- CRS/2022-23/04/912/UGC-DAE Consortium for Scientific Research, University Grants Commission
- CRS/2022-23/04/912/UGC-DAE Consortium for Scientific Research, University Grants Commission
- CRS/2022-23/04/912/UGC-DAE Consortium for Scientific Research, University Grants Commission
- RSP2024R304/King Saud University
LinkOut - more resources
Full Text Sources

