Cryopreserved human alternatively activated macrophages promote resolution of acetaminophen-induced liver injury in mouse
- PMID: 39843512
- PMCID: PMC11754469
- DOI: 10.1038/s41536-025-00393-3
Cryopreserved human alternatively activated macrophages promote resolution of acetaminophen-induced liver injury in mouse
Abstract
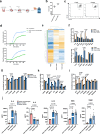
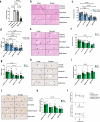
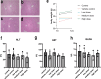
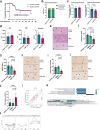
Acute liver failure is a rapidly progressing, life-threatening condition most commonly caused by an overdose of acetaminophen (paracetamol). The antidote, N-acetylcysteine (NAC), has limited efficacy when liver injury is established. If acute liver damage is severe, liver failure can rapidly develop with associated high mortality rates. We have previously demonstrated that alternatively, activated macrophages are a potential therapeutic option to reverse acute liver injury in pre-clinical models. In this paper, we present data using cryopreserved human alternatively activated macrophages (hAAMs)-which represent a potential, rapidly available treatment suitable for use in the acute setting. In a mouse model of APAP-induced injury, peripherally injected cryopreserved hAAMs reduced liver necrosis, modulated inflammatory responses, and enhanced liver regeneration. hAAMs were effective even when administered after the therapeutic window for NAC. This cell therapy approach represents a potential treatment for APAP overdose when NAC is ineffective because liver injury is established.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: S.J.F. and P.J.S.L. are founders of Resolution Therapeutics, which is developing a macrophage cell therapy product to treat patients at risk of liver decompensation; A.M.K. is a consultant to Resolution Therapeutics. The remaining authors declare no competing interests.
Figures

References
-
- Lewis, J. H. Drug-induced and toxic liver disease. In: Handbook of Liver Disease. 10.1016/B978-0-323-47874-8.00010-9 (2018).
-
- Tanne, J. Paracetamol causes most liver failure in UK and US. BMJ332, 628.2 (2006). - DOI
-
- Plevris, J. N., Schina, M. & Hayes, P. C. Review article: the management of acute liver failure. Aliment. Pharmacol. Ther.12, 405–418 (1998). - PubMed
-
- Lancaster, E. M., Hiatt, J. R. & Zarrinpar, A. Acetaminophen hepatotoxicity: an updated review. Arch. Toxicol. 89, 193–199 (2015). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

