Klebsiella pneumoniae employs a type VI secretion system to overcome microbiota-mediated colonization resistance
- PMID: 39843522
- PMCID: PMC11754592
- DOI: 10.1038/s41467-025-56309-8
Klebsiella pneumoniae employs a type VI secretion system to overcome microbiota-mediated colonization resistance
Abstract
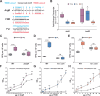
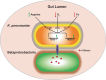
Microbial species must compete for space and nutrients to persist in the gastrointestinal (GI) tract, and our understanding of the complex pathobiont-microbiota interactions is far from complete. Klebsiella pneumoniae, a problematic, often drug-resistant nosocomial pathogen, can colonize the GI tract asymptomatically, serving as an infection reservoir. To provide insight on how K. pneumoniae interacts with the resident gut microbiome, we conduct a transposon mutagenesis screen using a murine model of GI colonization with an intact microbiota. Among the genes identified were those encoding a type VI secretion system (T6SS), which mediates contact-dependent killing of gram-negative bacteria. From several approaches, we demonstrate that the T6SS is critical for K. pneumoniae gut colonization. Metagenomics and in vitro killing assays reveal that K. pneumoniae reduces Betaproteobacteria species in a T6SS-dependent manner, thus identifying specific species targeted by K. pneumoniae. We further show that T6SS gene expression is controlled by several transcriptional regulators and that expression only occurs in vitro under conditions that mimic the gut environment. By enabling K. pneumoniae to thrive in the gut, the T6SS indirectly contributes to the pathogenic potential of this organism. These observations advance our molecular understanding of how K. pneumoniae successfully colonizes the GI tract.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures





References
MeSH terms
Substances
Grants and funding
- AI166642/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- R21 AI166642/AI/NIAID NIH HHS/United States
- T32 AI007151/AI/NIAID NIH HHS/United States
- T32 AI007401/AI/NIAID NIH HHS/United States
- AI178595/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

