Spinal cord injury induces transient activation of hepatic stellate cells in rat liver
- PMID: 39843526
- PMCID: PMC11754611
- DOI: 10.1038/s41598-025-87131-3
Spinal cord injury induces transient activation of hepatic stellate cells in rat liver
Abstract
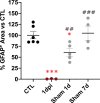
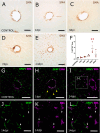
Spinal cord injury (SCI) causes abnormal liver function, the development of metabolic dysfunction-associated steatotic liver disease features and metabolic impairment in patients. Experimental models also demonstrate acute and chronic changes in the liver that may, in turn, affect SCI recovery. These changes have collectively been proposed to contribute to the development of a SCI-induced metabolic dysfunction-associated steatohepatitis (MASH). However, none of the existent studies have focused on hepatic stellate cells (HSCs), liver resident cells that are the primary drivers of collagen deposition and fibrosis following sustained liver damage. Here, we describe the transient activation of HSCs after a thoracic contusion in rats, considered a clinically relevant model of experimental SCI. We studied HSC during the time course of SCI, from 1 to 45 days post injury. We found a transient activation of HSCs after SCI, beginning with the acute downregulation of Glial Fibrillar Acidic Protein 1dpi. This is followed by a morphological and phenotypical transformation into alpha-smooth muscle actin (ACTA2/SMA) immunoreactive myofibroblast-like cells, peaking at 14 days post-injury and returning to control-like levels at later timepoints (45 days post-injury). These changes are not accompanied by fibrosis development but collagen deposition in peri-portal areas is observed at 45 days.
Keywords: Fibrosis; GFAP; Hepatic stellate cells; Liver; SMA; Spinal cord.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures


Similar articles
-
Analysis of glial fibrillary acidic protein (GFAP)-expressing ductular cells in a rat liver cirrhosis model induced by repeated injections of thioacetamide (TAA).Exp Mol Pathol. 2015 Jun;98(3):476-85. doi: 10.1016/j.yexmp.2015.03.010. Epub 2015 Mar 7. Exp Mol Pathol. 2015. PMID: 25758201
-
Characterization of glial fibrillary acidic protein (GFAP)-expressing hepatic stellate cells and myofibroblasts in thioacetamide (TAA)-induced rat liver injury.Exp Toxicol Pathol. 2013 Nov;65(7-8):1159-71. doi: 10.1016/j.etp.2013.05.008. Epub 2013 Jun 25. Exp Toxicol Pathol. 2013. PMID: 23806769
-
Spinal cord injury causes chronic liver pathology in rats.J Neurotrauma. 2015 Feb 1;32(3):159-69. doi: 10.1089/neu.2014.3497. Epub 2014 Oct 21. J Neurotrauma. 2015. PMID: 25036371 Free PMC article.
-
Glial fibrillary acidic protein as an early marker of hepatic stellate cell activation in chronic and posttransplant recurrent hepatitis C.Liver Transpl. 2008 Jun;14(6):806-14. doi: 10.1002/lt.21436. Liver Transpl. 2008. PMID: 18508359
-
MicroRNA-30a Suppresses the Activation of Hepatic Stellate Cells by Inhibiting Epithelial-to-Mesenchymal Transition.Cell Physiol Biochem. 2018;46(1):82-92. doi: 10.1159/000488411. Epub 2018 Mar 20. Cell Physiol Biochem. 2018. PMID: 29587268
References
-
- Goodus, M. T. & McTigue, D. M. Hepatic dysfunction after spinal cord injury: a vicious cycle of central and peripheral pathology? Exp. Neurol.325, 113160 (2020). - PubMed
-
- Campbell, S. J. et al. Liver kupffer cells control the magnitude of the inflammatory response in the injured brain and spinal cord. Neuropharmacology55, 780–787 (2008). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

