Antibiotic ecotoxicity and resistance risks in resource-constrained chicken and pig farming environments
- PMID: 39843536
- PMCID: PMC11721419
- DOI: 10.1038/s44259-024-00071-2
Antibiotic ecotoxicity and resistance risks in resource-constrained chicken and pig farming environments
Abstract
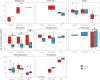
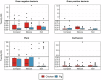
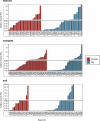
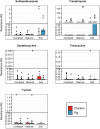
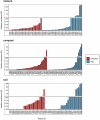
Antimicrobial resistance (AMR) data from agroecosystems in low- and middle-income countries is limited. We surveyed chicken (n = 52) and pig (n = 47) farms in Kenya to understand AMR in animal-environment pathways. Using LC-MS/MS, we validated the methods for analyzing eight common antibiotics and quantified the associated risks. Chicken compost (25.8%, n = 97/376) had the highest antibiotics prevalence, followed by pig manure-fertilized soils (23.1%, n = 83/360). The average antibiotic concentration was 63.4 µg/kg, which is below the environmentally relevant threshold (100 µg/kg), except for trimethoprim (221.4 µg/kg) among antibiotics and pig manure-fertilized soils (129.3 µg/kg) across sample types. Similarly, the average AMR risk quotient (RQ) was low (RQ < 0.1), except for trimethoprim and sulfamethoxazole (RQ ≥ 1). Ecotoxicity and AMR risks increased with flock size and the number of antibiotics used by pigs. Continuous environmental monitoring and large-scale studies on antibiotic contamination are crucial for evidence-based pollution control and the effective mitigation of environmental AMR.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures
References
-
- Ikhimiukor, O. O., Odih, E. E., Donado-Godoy, P. & Okeke, I. N. A bottom-up view of antimicrobial resistance transmission in developing countries. Nat. Microbiol.7, 757–765 (2022). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

