Real life outcome analysis of breast cancer brain metastases treated with Trastuzumab Deruxtecan
- PMID: 39843642
- PMCID: PMC11754752
- DOI: 10.1038/s41698-025-00801-3
Real life outcome analysis of breast cancer brain metastases treated with Trastuzumab Deruxtecan
Abstract
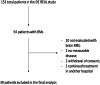
Tumor dissemination to the central nervous system (CNS) is almost a rule in the treatment journey of advanced HER2+ breast cancer (BC). Recent results demonstrated high intracranial efficacy with Trastuzumab Deruxtecan (T-DXd). However, a real-world evidence is lacking in literature. We conducted a multicenter, observational, retrospective real-world analysis on 39 cases collected at 12 Italian Oncological Units. Patients with brain metastases (BMs) from HER2 + BC treated with T-DXd in various treatment lines were enrolled. Primary endpoint was the intracranial overall response rate (iORR). Secondary endpoints were intra- and global progression free survival (iPFS - gPFS); other secondary objectives were the intracranial disease control rate (iDCR), duration of response (iDoR), clinical benefit rate at 6 and 12 months (iCBr), overall survival, and safety. iORR was 59%, iPFS was 15.6 months, gPFS was 11.8 months. iDCR was 94.9%, iDoR was 11.9 months, and iCBr at 6 and 12 months were 69.2% and 59%, respectively. OS was not reached, with an overall rate of 77.9% of patients alive at 12 months. This study confirmed the high intracranial efficacy and manageable safety profile of T-DXd in this first-ever real world analysis.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

References
-
- Hurvitz, S. A. et al. Central nervous system metastasis in patients with HER2-positive metastatic breast cancer: patient characteristics, treatment, and survival from SystHERs. Clin. Cancer Res.25, 2433–2441 (2019). - PubMed
-
- Steeg P. S. The blood-tumour barrier in cancer biology and therapy. Nat. Rev. Clin. Oncol. 18, 696–714 (2021). - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

