Synergistic combination of orally available safe-in-man pleconaril, AG7404, and mindeudesivir inhibits enterovirus infections in human cell and organoid cultures
- PMID: 39843710
- PMCID: PMC11754576
- DOI: 10.1007/s00018-025-05581-4
Synergistic combination of orally available safe-in-man pleconaril, AG7404, and mindeudesivir inhibits enterovirus infections in human cell and organoid cultures
Abstract
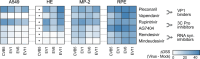
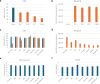
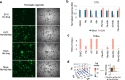
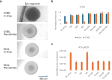
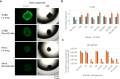
Enteroviruses can infect various human organs, causing diseases such as meningitis, the common cold, hand-foot-and-mouth disease, myocarditis, pancreatitis, hepatitis, poliomyelitis, sepsis, and type 1 diabetes. Currently, there are no approved treatments for enterovirus infections. In this study, we identified a synergistic combination of orally available, safe-in-man pleconaril, AG7404, and mindeudesivir, that at non-toxic concentrations effectively inhibited enterovirus replication in human cell and organoid cultures. Importantly, the cocktail did not alter glucose and insulin levels in the culture medium of pancreatic β-cells and preserved the contraction rhythm of infected heart organoids. These findings highlight a promising drug cocktail for further preclinical studies and clinical trials targeting a broad range of enterovirus-mediated diseases.
Keywords: Antiviral drug combination; Broad-spectrum antivirals; Drug synergy; Enterovirus.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval: The study involved commercially available human cell lines, WT virus strains, which we isolated from patient samples or obtained via collaborations. All experiments with viruses were performed in BSL2 laboratories in compliance with the guidelines of the national authorities, and under appropriate ethical and safety approvals. All experiments conducted with pancreatic cancer organoids have been approved by the Helsinki University Hospital ethical board, HUS/74/2023, HUS/23/2024. The Medical Ethical Committee of the Erasmus MC Rotterdam granted permission for study on lung organoids (METC 2012 − 512). Competing interests: The authors have no relevant financial or non-financial interests to disclose.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

