Tissue-resident memory CD8 T cell diversity is spatiotemporally imprinted
- PMID: 39843748
- PMCID: PMC11903307
- DOI: 10.1038/s41586-024-08466-x
Tissue-resident memory CD8 T cell diversity is spatiotemporally imprinted
Abstract
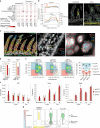
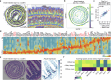
Tissue-resident memory CD8 T (TRM) cells provide protection from infection at barrier sites. In the small intestine, TRM cells are found in at least two distinct subpopulations: one with higher expression of effector molecules and another with greater memory potential1. However, the origins of this diversity remain unknown. Here we proposed that distinct tissue niches drive the phenotypic heterogeneity of TRM cells. To test this, we leveraged spatial transcriptomics of human samples, a mouse model of acute systemic viral infection and a newly established strategy for pooled optically encoded gene perturbations to profile the locations, interactions and transcriptomes of pathogen-specific TRM cell differentiation at single-transcript resolution. We developed computational approaches to capture cellular locations along three anatomical axes of the small intestine and to visualize the spatiotemporal distribution of cell types and gene expression. Our study reveals that the regionalized signalling of the intestinal architecture supports two distinct TRM cell states: differentiated TRM cells and progenitor-like TRM cells, located in the upper villus and lower villus, respectively. This diversity is mediated by distinct ligand-receptor activities, cytokine gradients and specialized cellular contacts. Blocking TGFβ or CXCL9 and CXCL10 sensing by antigen-specific CD8 T cells revealed a model consistent with anatomically delineated, early fate specification. Ultimately, our framework for the study of tissue immune networks reveals that T cell location and functional state are fundamentally intertwined.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: M.R.-C. is a co-founder, scientific adviser and board member of TCura Bioscience, Inc. A.F. is a co-founder, CEO and board member of TCura Bioscience. B.B. receives consulting fees from Bristol Myers Squibb and Pfizer and research grants from Merck and Gilead. A.W.G. is a co-founder of TCura Bioscience, Inc. and serves on the scientific advisory board of ArsenalBio and Foundery Innovations. The other authors declare no competing interests.
Figures












Update of
-
Functional Diversity of Memory CD8 T Cells is Spatiotemporally Imprinted.bioRxiv [Preprint]. 2024 Mar 25:2024.03.20.585130. doi: 10.1101/2024.03.20.585130. bioRxiv. 2024. Update in: Nature. 2025 Mar;639(8054):483-492. doi: 10.1038/s41586-024-08466-x. PMID: 38585842 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

