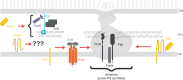
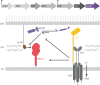
The Tol Pal system integrates maintenance of the three layered cell envelope
- PMID: 39843782
- PMCID: PMC11721397
- DOI: 10.1038/s44259-024-00065-0
The Tol Pal system integrates maintenance of the three layered cell envelope
Abstract
The rapid emergence of antibiotic-resistant superbugs poses a significant global health threat. Gram-negative bacteria are the primary culprits due to their robust, tripartite cell envelope. This review explores the emerging role of the trans-envelope Tol-Pal system in maintaining envelope integrity, by connecting envelope layers and serving as a protein interaction hub. Targeting the Tol-Pal system offers a promising approach for the development of novel envelope-disrupting antimicrobials.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

References
-
- Walsh, C. Molecular mechanisms that confer antibacterial drug resistance. Nature406, 775–781 (2000). - PubMed
-
- O'neill, J. I. M. Antimicrobial resistance: tackling a crisis for the health and wealth of nations. Rev. Antimicrob. Resist. (2014).
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

