Empowering African Expertise: Enhancing Safety Data Integration and Signal Detection for COVID-19 Vaccines Through the African Union Smart Safety Surveillance Joint Signal Management Group
- PMID: 39843797
- PMCID: PMC11829835
- DOI: 10.1007/s40264-024-01493-7
Empowering African Expertise: Enhancing Safety Data Integration and Signal Detection for COVID-19 Vaccines Through the African Union Smart Safety Surveillance Joint Signal Management Group
Abstract
Introduction: The COVID-19 pandemic accelerated new vaccine development. Limited safety data necessitated robust global safety surveillance to accurately identify and promptly communicate potential safety issues. The African Union Smart Safety Surveillance (AU-3S) program established the Joint Signal Management (JSM) group to support identification of potential vaccine safety concerns in five pilot countries (Ethiopia, Ghana, Kenya, Nigeria, South Africa), accounting for approximately 35% of the African population.
Objective: Our objective was to provide an overview of the JSM group's role in supporting signal management activities for the AU-3S program during the COVID-19 pandemic.
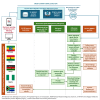
Methods: Spontaneous, electronically reported COVID-19 vaccine adverse events following immunization (AEFI) from each country's safety data were integrated into the interim Data Integration and Signal Detection system. Statistical disproportionality methods were used to identify and review vaccine-event combinations (VECs) for potential safety concerns. The JSM group-which comprised pharmacovigilance and subject matter experts from National Medicine Regulatory Authorities, Expanded Programs on Immunization, and vaccine safety committees-conducted signal detection activities on cross-country safety data and provided recommendations.
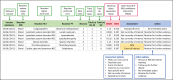
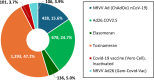
Results: From April 2021 to December 2023, a total of 48,294 spontaneously reported AEFI were analyzed for six COVID-19 vaccines (NRVV Ad [ChAdOx1 nCoV-19]; Ad26.COV2.S; Elasomeran; Tozinameran; Covid-19 vaccine [Vero Cell], Inactivated; NRVV Ad26 [Gam-Covid-Vac]) administered in Ethiopia (34.6%), Nigeria (30.3%), South Africa (16.9%), Ghana (13.5%), and Kenya (4.7%). Overall, 2,742 VECs were validated. A causal association between the COVID-19 vaccines and the reported AEFI cannot be inferred, as data were reported spontaneously. JSM group recommendations included monitoring for further evidence, no immediate action required, engaging marketing authorization holder(s) for additional information, or sensitizing healthcare providers and/or the public about events. Although no new safety signals were identified, nine safety-related recommendations were issued, including patient and healthcare provider education.
Conclusions: The JSM group established a scalable and replicable model for future signal management of other priority health products in low- and middle-income countries, fostering ongoing collaboration and capacity building. Knowledge and experience gained from this pilot initiative will guide stakeholders in future safety surveillance initiatives within the African continent.
Plain language summary
During the COVID-19 pandemic, the urgent need for vaccines led to the development of new vaccines. However, there were concerns about the limited amount of safety data on the vaccines when used in the real world by large populations. To address this, the African Union launched its Smart Safety Surveillance program in five countries in Africa. Each of these countries used mobile technology for spontaneous reporting of COVID-19 vaccine adverse events following immunization (AEFI), resulting in a huge amount of safety data combined in one database. This allowed experts in Africa from various fields (i.e., the Joint Signal Management [JSM] group) to use statistical methods to analyze the combined data and identify any safety concerns. Over 32 months, the JSM group analyzed 48,294 spontaneously reported AEFI for six COVID-19 vaccines and verified the safety of 2,742 events reported for specific vaccines. However, a causal association with the COVID-19 vaccines cannot be assumed, because AEFI were reported spontaneously and were not investigated. This notwithstanding, the JSM group was able to make recommendations to the five countries, including education for patients and healthcare providers and engaging with vaccine manufacturers for more information on specific events. This initiative established a unified collaborative safety response model for low- and middle-income countries that is also applicable to other health products and diseases in Africa beyond COVID-19 vaccines. The JSM group seeks to provide reliable guidance for future safety surveillance initiatives in Africa.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Funding: Open access funding provided by Sefako Makgatho Health Sciences University. The implementation of the AU-3S program in the pilot countries, which resulted in the work presented in this paper, were funded by the Bill & Melinda Gates Foundation, Seattle, WA, USA (grant number: INV-045215 [OPP1213055]). Open access for this paper was enabled through an agreement between Sefako Makgatho Health Sciences University and Springer Nature. Conflict of interest: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Availability of data and material: N/A. Ethics approval: N/A. Consent to participate: N/A. Consent for publication: All authors have read the manuscript and consent to its publication. Code availability: N/A. Author contributions: JSM group involved in the implementation of the work presented in this paper: VPD, HMG, MBA, NYB, MB, GTS, JOG, EMG, TA, WA, HJL, MCL, AM, KAA, MM, UGE, HM, AKN, MS, MK, KM, YlT, AA, KMY, OE, LS, KD, KO, MCA, DMD, HG, BS, FS, AA, and JCM. Data acquisition and analysis: VPN, MBA, NYB, and HM. First draft of the manuscript: VPN and JCM. Revision for important intellectual content: VPD, HMG, MBA, NYB, MB, GTS, JOG, EMG, TA, WA, HJL, MCL, AM, KAA, MM, UGE, HM, AKN, MS, MK, KM, YlT, AA, KMY, OE, LS, KD, KO, MCA, DMD, HG, BS, FS, AA, and JCM. All authors have read and approved the final version of the manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to accuracy or integrity of any part of the work are appropriately investigated and resolved.
Figures


References
-
- World Health Organization. The importance of pharmacovigilance. Safety Monitoring of medicinal products. 2002 [cited 2002 1 January 2002]. https://www.who.int/publications/i/item/10665-42493.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

