Synthesis, H2S releasing properties, antiviral and antioxidant activities and acute cardiac effects of nucleoside 5'-dithioacetates
- PMID: 39843902
- PMCID: PMC11754443
- DOI: 10.1038/s41598-025-85351-1
Synthesis, H2S releasing properties, antiviral and antioxidant activities and acute cardiac effects of nucleoside 5'-dithioacetates
Abstract
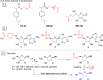
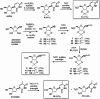
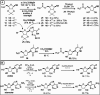
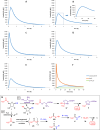
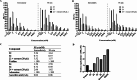
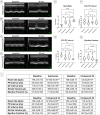
Hydrogen sulfide (H2S) is an endogenous gasotransmitter with cardioprotective and antiviral effects. In this work, new cysteine-selective nucleoside-H2S-donor hybrid molecules were prepared by conjugating nucleoside biomolecules with a thiol-activatable dithioacetyl group. 5'-Dithioacetate derivatives were synthesized from the canonical nucleosides (uridine, adenosine, cytidine, guanosine and thymidine), and the putative 5'-thio metabolites were also produced from uridine and adenosine. According to our measurements made with an H2S-specific sensor, nucleoside dithioacetates are moderately fast H2S donors, the guanosine derivative showed the fastest kinetics and the adenosine derivative the slowest. The antioxidant activity of 5'-thionucleosides is significantly higher than that of trolox, but lower than that of ascorbic acid, while intact dithioacetates have no remarkable antioxidant effect. In human Calu cells, the guanosine derivative showed a moderate anti-SARS-CoV-2 effect which was also confirmed by virus yield reduction assay. Dithioacetyl-adenosine and its metabolite showed similar acute cardiac effects as adenosine, however, it is noteworthy that both 5'-thio modified adenosines increased left ventricular ejection fraction or stroke volume, which was not observed with native adenosine.
Keywords: Antioxidant; Antiviral; Dithioacetate; H2S donor; H2S release kinetics; Nucleoside; SARS-CoV-2.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures

References
-
- Baskar, R. & Bian, J. Hydrogen sulfide gas has cell growth regulatory role. Eur. J. Pharmacol.656, 5–9. 10.1016/j.ejphar.2011.01.052 (2011). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

