Development and functional evaluation of a psoralen-conjugated nucleoside mimic for triplex-forming oligonucleotides
- PMID: 39843926
- PMCID: PMC11754458
- DOI: 10.1038/s42004-025-01416-2
Development and functional evaluation of a psoralen-conjugated nucleoside mimic for triplex-forming oligonucleotides
Abstract
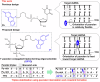
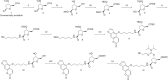
Psoralen-conjugated triplex-forming oligonucleotides (Ps-TFOs) have been employed for the photodynamic regulation of gene expression by the photo-cross-linking of psoralen with the target DNA. However, stable triplex formation requires a consecutive purine base sequence in one strand of the target DNA duplexes. The pyrimidine-base interruption in the consecutive purine base sequence drastically decreases the thermodynamic stability of the corresponding triplex, which hampers the TFO application. Here, we propose a design of the Ps-TFO for stable triplex formation with target DNA sequences containing pyrimidine-base interruptions under physiological conditions. This Ps-TFO, named 1'(one)-psoralen-conjugated triplex-forming oligonucleotide (OPTO), incorporates a synthesized nucleoside mimic 1'-psoralen-conjugated deoxyribose to increase the thermodynamic stability of the corresponding triplex by the intercalation of psoralen. The triplex-forming abilities of the OPTO were successfully demonstrated in combination with LNA and 5-methylcytosine, indicating that the use of OPTO will expand the range of the target sequences of TFO for photodynamic gene regulation.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures





References
-
- Karympalis, V., Kalopita, K., Zarros, A. & Carageorgiou, H. Regulation of gene expression via triple helical formations. Biochemistry69, 855–860 (2004). - PubMed
-
- Moser, H. E. & Dervan, P. B. Sequence-specific cleavage of double helical DNA by triple helix formation. Science238, 645–650 (1987). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

