Treadmill exercise prevents stress-induced anxiety-like behaviors via enhancing the excitatory input from the primary motor cortex to the thalamocortical circuit
- PMID: 39843934
- PMCID: PMC11754434
- DOI: 10.1038/s41467-025-56258-2
Treadmill exercise prevents stress-induced anxiety-like behaviors via enhancing the excitatory input from the primary motor cortex to the thalamocortical circuit
Abstract
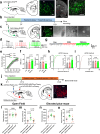
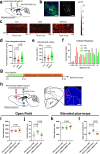
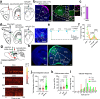
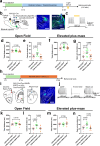
Physical exercise effectively prevents anxiety disorders caused by environmental stress. The neural circuitry mechanism, however, remains incomplete. Here, we identified a previously unrecognized pathway originating from the primary motor cortex (M1) to medial prefrontal cortex (mPFC) via the ventromedial thalamic (VM) nuclei in male mice. Besides anatomical evidence, both ex vivo and in vivo recordings showed enhanced excitability of M1-VM inputs to the prelimbic (PrL) region of mPFC upon 14-day treadmill exercise on a chronic restraint stress (CRS) mouse model. Further functional interrogations demonstrated that the activation of this neural circuit is both necessary and sufficient to direct the anxiolytic effect of exercise training in CRS mice. Our findings provide more insights into the neural circuits connecting motor and mental regions under exercise paradigm and implicate potential targets for neuromodulation in treating anxiety disorders.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



Similar articles
-
A Prelimbic Cortex-Thalamus Circuit Bidirectionally Regulates Innate and Stress-Induced Anxiety-Like Behavior.J Neurosci. 2024 Jul 17;44(29):e2103232024. doi: 10.1523/JNEUROSCI.2103-23.2024. J Neurosci. 2024. PMID: 38886059 Free PMC article.
-
Anterolateral Motor Cortex Connects with a Medial Subdivision of Ventromedial Thalamus through Cell Type-Specific Circuits, Forming an Excitatory Thalamo-Cortico-Thalamic Loop via Layer 1 Apical Tuft Dendrites of Layer 5B Pyramidal Tract Type Neurons.J Neurosci. 2018 Oct 10;38(41):8787-8797. doi: 10.1523/JNEUROSCI.1333-18.2018. Epub 2018 Aug 24. J Neurosci. 2018. PMID: 30143573 Free PMC article.
-
Medial prefrontal cortex-periaqueductal gray circuit overcomes anxiety-like behavior in male mice following adversity.J Affect Disord. 2025 Mar 1;372:149-159. doi: 10.1016/j.jad.2024.12.017. Epub 2024 Dec 3. J Affect Disord. 2025. PMID: 39638057
-
Thalamic afferents to prefrontal cortices from ventral motor nuclei in decision-making.Eur J Neurosci. 2019 Mar;49(5):646-657. doi: 10.1111/ejn.14215. Epub 2018 Dec 3. Eur J Neurosci. 2019. PMID: 30346073 Free PMC article. Review.
-
Stress-protective neural circuits: not all roads lead through the prefrontal cortex.Stress. 2014 Jan;17(1):1-12. doi: 10.3109/10253890.2013.794450. Epub 2013 Jun 4. Stress. 2014. PMID: 23574145 Review.
Cited by
-
Neural Circuit Mapping and Neurotherapy-Based Strategies.Cell Mol Neurobiol. 2025 Jul 26;45(1):75. doi: 10.1007/s10571-025-01595-5. Cell Mol Neurobiol. 2025. PMID: 40715588 Free PMC article. Review.
References
-
- Tyrer, P. & Baldwin, D. Generalised anxiety disorder. Lancet368, 2156–2166 (2006). - PubMed
-
- Villagrasa, B. et al. Prevalence of anxiety disorder among older adults in Spain: a meta-analysis. J. Affect Disord.246, 408–417 (2019). - PubMed
-
- Aune, T., Nordahl, H. M. & Beidel, D. C. Social anxiety disorder in adolescents: prevalence and subtypes in the Young-HUNT3 study. J. Anxiety Disord.87, 102546 (2022). - PubMed
-
- Bosman, R. C., et al., Prevalence and course of subthreshold anxiety disorder in the general population: A three-year follow-up study. J. Affect Disord. 247, 105–113 (2019). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

