Assessment of tuberculosis drug efficacy using preclinical animal models and in vitro predictive techniques
- PMID: 39843983
- PMCID: PMC11721416
- DOI: 10.1038/s44259-024-00066-z
Assessment of tuberculosis drug efficacy using preclinical animal models and in vitro predictive techniques
Abstract
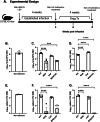
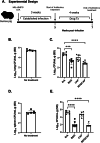
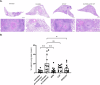
Tuberculosis (TB) killed approximately 1.3 million people in 2022 and remains a leading cause of death from the bacteria Mycobacterium tuberculosis (M.tb); this number of deaths was surpassed only by COVID-19, caused by the SARS-CoV-2 virus. The alarming emergence of multidrug-resistant (MDR) and extensively drug-resistant (XDR) M.tb strains presents an urgent need for effective new treatments. Our study aimed to determine the synergistic effects of antibiotic combinations against M.tb. Using a high-throughput in vitro checkerboard assay, we evaluated the interactions of Bedaquiline (BDQ) and other antibiotics including Capreomycin (CAP), Linezolid (LIN), and Sutezolid (SUT) against M.tb H37Rv. BDQ and CAP demonstrated in vitro enhanced effect, which prompted further investigation in vivo using the murine low dose aerosol (LDA) model. After aerosol challenge with M.tb, C57BL/6 mice were treated with BDQ, CAP, or their combination, starting 28 days post-infection. The antimicrobial treatment lasted four weeks, and the bacterial burden in lung and spleen tissues was assessed at the end of treatment. At 4 weeks post-treatment, a significant reduction in bacterial load was observed within the lungs and spleens of mice given BDQ alone or given as a BDQ/CAP combination compared to the untreated group. In contrast, CAP monotherapy led to an increase in bacterial load within the lung and no significant difference in bacterial burden in the spleen in comparison to the untreated mice. These results were confirmed in the guinea pig model of TB, where both BDQ and the BDQ/CAP combination treatment led to a decrease in bacterial burden in the lung and spleen, whereas CAP had no significant effect on bacterial burden at the 4-week post treatment timepoint. We next determined whether there may be differences in vitro with the BDQ/CAP combination against M.tb lineages 1, 2 and 4. We determined that in vitro enhanced effect was not observed in some representative strains of M.tb lineage 4, indicating variability in drug effectiveness across M.tb lineages. This research underscores the complexity of TB treatment and the critical need for innovative approaches to combat this global health threat.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures


References
LinkOut - more resources
Full Text Sources
Miscellaneous

