Unconventional use of injectable long-acting cabotegravir and rilpivirine against HIV-1 in PWH in clinical need: 52 weeks real-world data
- PMID: 39844075
- PMCID: PMC11756072
- DOI: 10.1186/s12879-025-10499-0
Unconventional use of injectable long-acting cabotegravir and rilpivirine against HIV-1 in PWH in clinical need: 52 weeks real-world data
Abstract
Background: Long-acting Cabotegravir and Rilpivirine (LA CAB + RPV) shows potential advantages in heavily comorbid and even in viremic people with HIV (PWH). We assessed LA CAB + RPV durability in a cohort of PWH with a high comorbidity burden and adherence issues.
Methods: Retrospective observational study in two Italian outpatient settings enrolling PWH who switched to LA CAB + RPV from February 2021 to January 2024 in presence of exclusion criteria enlisted in registrational trials or with other worrisome clinical risks. Kaplan-Meier (KM) was used to assess the probability of CAB/RPV discontinuation. Cox regression analysis was used to evaluate potential predictors of discontinuation.
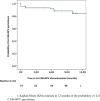
Results: We enrolled 74 PWH, with a median of 7 injections (IQR 5-9), a median age of 53 years (IQR 45-61), median time of exposure to antiretrovirals of 11 years (IQR 8-18) and median time from HIV diagnosis of 11.8 years (IQR 6.6-18.2). Eleven (14.9%) discontinued LA CAB + RPV mainly for injection-site pain. Of 53 PWH who were undetectable before switch, 37 maintained viral suppression at week 52. We registered only one virological failure at week 12. Twenty-one started injections with unsuppressed viral loads (median 66 cps/ml, IQR 40-215) and 10 (47.6%) achieved viral suppression. Overall probability of discontinuation was 14.9% at week 52. Younger age was protective against discontinuation (HR 0.93, 95%CI 0.88-0.99, p = 0.048).
Conclusions: Our results support the potential advantages in using LA CAB + RPV in PWH with adherence issues and comorbidities.
Keywords: Adherence issues; Comorbidities; Long acting antiretrovirals; Population in need; Virological suppression.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was conducted according to the Helsinki declaration. All data used in the study were previously anonymized, according to the requirements of the Italian Data Protection Code (leg. Decree 196/2003) and by the general authorizations issued by the Data Protection Authority. Informed consent was obtained from all participants, and it was approved Institutional Review Board (IRB) of the ASST Grande Ospedale Metropolitano Niguarda, Milan, Italy, and administered according to internal procedures. Our study was submitted to the Institutional Review Board (IRB) of the ASST Grande Ospedale Metropolitano Niguarda, Milan, Italy and received the ethical approval for the unconventional and off-label use of this long-acting strategy. Protocol number: [ID 5370– approval number 5370_16.10.2024_N bis]. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
References
-
- Rusconi S, Marcotullio S, Cingolani A. Long-acting agents for HIV infection: biological aspects, role in treatment and prevention, and patient’s perspective. New Microbiol. 2017;40(2):75–9. - PubMed