Multiple time points for detecting circulating tumor DNA to monitor the response to neoadjuvant therapy in breast cancer: a meta-analysis
- PMID: 39844103
- PMCID: PMC11752932
- DOI: 10.1186/s12885-025-13526-0
Multiple time points for detecting circulating tumor DNA to monitor the response to neoadjuvant therapy in breast cancer: a meta-analysis
Abstract
Background: Not all breast cancer (BC) patients can benefit from neoadjuvant therapy (NAT). A poor response may result in patients missing the best opportunity for treatment, ultimately leading to a poor prognosis. Thus, to identify an effective predictor that can assess and predict patient response at early time points, we focused on circulating tumor DNA (ctDNA), which is a vital noninvasive liquid biopsy biomarker. We performed a meta-analysis to explore the predictive value of response by monitoring ctDNA at four time points of NAT using pathologic complete response (pCR) and residual cancer burden (RCB).
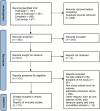
Methods: By searching Embase, PubMed, the Cochrane Library, and the Web of Science until December 24, 2023, we selected studies concerning the relationship between ctDNA and response or prognosis. We analysed the results at the following various time points: baseline (T0), first cycle of NAT (T1), mid-treatment (MT), and end of NAT (EOT). pCR and RCB were used to evaluate the response as the primary endpoint. The secondary endpoint was to investigate the relationship between ctDNA and prognosis. Odds ratios (ORs) and hazard ratios (HRs) were used as effect indicators.
Results: Thirteen reports from twelve studies were eligible for inclusion in this meta-analysis. The results demonstrated that ctDNA negativity was associated with pCR at T1 (OR = 0.34; 95% CI: 0.21-0.57), MT (OR = 0.35; 95% CI: 0.20-0.60), and EOT (OR = 0.38; 95% CI: 0.22-0.66). When RCB was used to evaluate responses, ctDNA negativity was associated with RCB-0/I at the MT (OR = 0.34; 95% CI: 0.21-0.55) and EOT (OR = 0.26; 95% CI: 0.15-0.46). Furthermore, ctDNA positivity at T1 predicted a worse prognosis for patients (HR = 2.73; 95% CI: 1.29-5.75). We also performed a subgroup analysis to more accurately assess the predictive value of ctDNA for triple-negative breast cancer.
Conclusions: Our meta-analysis suggested that the ctDNA status at the early stage of NAT can predict patient response, which provides evidence for adjusting personalized treatment strategies and improving patient survival.
Prospero registration number: CRD42024496465.
Keywords: Biomarkers; Breast cancer; Circulating tumor DNA; Neoadjuvant therapy; Prognosis; Response.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: Not applicable. Competing interests: The authors declare no competing interests.
Figures


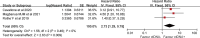
References
-
- I-SPY2 Trial Consortium, Yee D, DeMichele AM, Yau C, Isaacs C, Symmans WF, et al. Association of event-free and distant recurrence-free Survival with Individual-Level Pathologic Complete response in Neoadjuvant Treatment of Stages 2 and 3 breast Cancer: three-year follow-up analysis for the I-SPY2 adaptively randomized clinical trial. JAMA Oncol. 2020;6:1355–62. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 82203786/the National Natural Science Foundation of China
- 82373231/the National Natural Science Foundation of China
- 2023-BS-105/the Natural Science Foundation of Liaoning Province of China
- 2022-YGJC-68/the Natural Science Foundation of Liaoning Province of China
- CYBER-2022-001/Chinese Young Breast Experts Research Project
LinkOut - more resources
Full Text Sources
Medical

