Integrating BRCA testing into routine prostate cancer care: a multidisciplinary approach by SIUrO and other Italian Scientific Societies
- PMID: 39844131
- PMCID: PMC11756108
- DOI: 10.1186/s12885-025-13521-5
Integrating BRCA testing into routine prostate cancer care: a multidisciplinary approach by SIUrO and other Italian Scientific Societies
Abstract
Prostate cancer (PCa) ranks among the most prevalent malignancies in men, with notable associations to Hereditary Breast and Ovarian Cancer Syndrome (HBOC) and Lynch Syndrome, both linked to germline likely pathogenetic variant/pathogenetic variant (LPV/PV) in genes involved in DNA repair. Among these genes, BRCA2 in PCa patients is the most frequently altered. Despite progresses, challenges in BRCA carriers detection persist, with a quarter of PCa cases lacking family history.To address these challenges, a multidisciplinary expert panel from six Italian Scientific Societies, formulated a care pathway to integrate BRCA testing into routine clinical practice in different Italian geographical areas.The development process, promoted by the Italian Society of Uro-Oncology (SIUrO), involved three key stages. A preliminary meeting convened teams from different Italian regions to establish minimal requirements for a mini-counseling program (defined as a pre-test consultation carried out by clinicians responsible of patients' management) and propose care pathway models. At the same time, a comprehensive survey was launched to highlight regional variations in BRCA testing and identify eventual obstacles to testing activities. A subsequent meeting synthesized care pathway proposals and formulated a unified framework, acknowledging regional legislative variations as enriching factors. Lastly, implementation of the unified framework was promoted by the project faculty and identified regional team leaders.Survey results revealed significant regional disparities in BRCA testing, reimbursement policies, and access to genetic counseling. The proposed mini-counseling program outlined essential steps for patient identification, information provision, and multidisciplinary review, aiming to streamline BRCA testing processes.Expert recommendations emphasized offering tumor genetic testing to metastatic PCa patients eligible for PARP-i treatment and outlined criteria for genetic counseling and germline testing. Key considerations included family history and tumor characteristics.In conclusion, the proposed care pathway represents a critical step towards integrating BRCA testing into routine PCa care, aiming to optimize patient management and familial risk assessment within the constraints of the Italian healthcare system.
Keywords: BRCA; Care pathway; Genetic counseling; Mini-counseling; Prostate cancer.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. This manuscript does not report on or involve the use of any animal or human data or tissue. Consent for publication: Not applicable. This research does not involve human participants. Competing interests: A. Cimadamore: honoraria/ consulting fees by AstraZeneca/MSD; S. Bracarda: Advisory Board or Steering Committee Member for: Bayer, Astellas, Janssen, Pfizer, BMS, MSD, Novartis (AAA), Roche-Genentech, Ipsen, AstraZeneca, Merck, Gilead, Indicon, Genenta. Travel Accomodation by: MSD, Pfizer, Bayer. All other authors have declared no conflicts of interest.
Figures
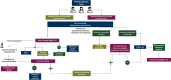
References
-
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. 10.3322/caac.21660. - PubMed
-
- Hemminki K. Familial risk and familial survival in prostate cancer. World J Urol. 2012;30(2):143–8. 10.1007/s00345-011-0801-1. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

