Knockout IL4I1 affects macrophages to improve poor efficacy of CD19 CAR-T combined with PD-1 inhibitor in relapsed/refractory diffuse large B-cell lymphoma
- PMID: 39844281
- PMCID: PMC11752997
- DOI: 10.1186/s12967-024-06028-3
Knockout IL4I1 affects macrophages to improve poor efficacy of CD19 CAR-T combined with PD-1 inhibitor in relapsed/refractory diffuse large B-cell lymphoma
Abstract
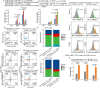
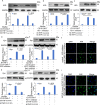
Chimeric antigen receptor (CAR) T-cell therapy plays a critical role in the treatment of B-cell hematologic malignancies. The combination of PD-1 inhibitors and CAR-T has shown encouraging results in treating patients with relapsed/refractory (R/R) diffuse large B-cell lymphoma (DLBCL). However, there are still cases where treatment is ineffective. This study aimed to investigate the role of IL4I1 in the poor efficacy of CD19 CAR-T combined with PD-1 inhibitors in R/R DLBCL and to explore potential mechanisms. Transcriptomic and metabolomic correlation analyses were performed on tumor tissue from DLBCL patients. We employed an in vitro co-culture system consisting of Pfeiffer cells, CD19 CAR-T and macrophages to investigate the underlying mechanisms. It was found that IL4I1 levels were significantly increased in the tumor tissues of R/R DLBCL patients compared to responders. Correlation analysis revealed a positive association between IL4I1 and tryptophan (Trp)-kynurenic acid (Kyn) related metabolites. In the in vitro co-culture model, the presence of IL4I1 inhibited the cytotoxicity of CAR-T cells. Depletion of IL4I1 disrupted the IDO-AHR-Kyn signaling pathway, thereby enhancing the effectiveness of PD-1 inhibitors in combination with CD19 CAR-T for DLBCL treatment. CAR-T-mediated cytotoxicity was significantly inhibited when IL4I1 was present in the in vitro co-culture model. These findings suggest that IL4I1 may be a contributing factor to poor prognosis in R/R DLBCL patients. IL4I1 expression enhances immunosuppression via the IDO-AHR-Kyn pathway, inhibiting the effectiveness of PD-1 inhibitors combined with CD19 CAR-T. Therefore, suppression of IL4I1 may represent a potential target for combination therapy in DLBCL.
Keywords: CD19 CAR-T; DLBCL; IL4I1; Metabolism; PD-1 inhibitor.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Informed consent was provided with lymphoma and healthy donors agreed to participate in this experiment within a clinical trial. Ethics approval was provided at the Department of Hematology at Tianjin First Central Hospital (Tianjin, China) (ChiCTR-ONN-16009862; Tianjin First Central Hospital Medical Ethics Committee). Competing interest: The authors declared no potential conflicts of interest concerning the research, authorship, and/or publication of this article.
Figures


Similar articles
-
An exploration of the initiation time and patient selection of PD-1 inhibitors/PD-1 inhibitors combined with chemotherapy as salvage therapy in R/R DLBCL patients after anti-CD19-CAR T-cell therapy.Cell Transplant. 2025 Jan-Dec;34:9636897251338713. doi: 10.1177/09636897251338713. Epub 2025 Jun 5. Cell Transplant. 2025. PMID: 40474425 Free PMC article.
-
Efficacy and safety of zanubrutinib combined with chimeric antigen receptor T-cell therapy targeting CD19 in refractory or relapsed diffuse large B cell lymphoma: A retrospective analysis.Cancer Treat Res Commun. 2025;43:100902. doi: 10.1016/j.ctarc.2025.100902. Epub 2025 Mar 29. Cancer Treat Res Commun. 2025. PMID: 40158266
-
In vitro functional validation of anti-CD19 chimeric antigen receptor T cells expressing lysine-specific demethylase 1 short hairpin RNA for the treatment of diffuse large B cell lymphoma.Front Immunol. 2025 Jan 13;15:1521778. doi: 10.3389/fimmu.2024.1521778. eCollection 2024. Front Immunol. 2025. PMID: 39872520 Free PMC article.
-
CD19 CAR-T cell therapy for relapsed or refractory diffuse large B cell lymphoma: Why does it fail?Semin Hematol. 2023 Nov;60(5):329-337. doi: 10.1053/j.seminhematol.2023.11.007. Epub 2023 Dec 5. Semin Hematol. 2023. PMID: 38336529 Free PMC article. Review.
-
Overcoming Barriers to Referral for Chimeric Antigen Receptor T Cell Therapy in Patients with Relapsed/Refractory Diffuse Large B Cell Lymphoma.Transplant Cell Ther. 2023 Jul;29(7):440-448. doi: 10.1016/j.jtct.2023.04.003. Epub 2023 Apr 7. Transplant Cell Ther. 2023. PMID: 37031747 Review.
Cited by
-
Molecular pathology of lymphoma and its treatment strategies: from mechanistic elucidation to precision medicine.Front Immunol. 2025 Jul 9;16:1620895. doi: 10.3389/fimmu.2025.1620895. eCollection 2025. Front Immunol. 2025. PMID: 40703518 Free PMC article. Review.
References
-
- Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–42. - PubMed
-
- Taylor RB, Paolo FC. A paradox of choice: sequencing therapy in relapsed/refractory diffuse large B-cell lymphoma. Blood Rev. 2024;63:101140. - PubMed
-
- Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-Cell lymphoma. N Engl J Med. 2019;380(1):45–56. - PubMed
MeSH terms
Substances
Grants and funding
- 81970180 to MZ/Innovative Research Group Project of the National Natural Science Foundation of China
- TJWJ2022QN030 to MZ/Tianjin Municipal Transportation Commission Science and Technology Development Plan Project
- 21JCZDJC01240/the Key projects of Tianjin Applied Basic Research and Multi-Investment Fund
- TJWJ2022XK018 to MZ/the Science and Technology Project of Tianjin Municipal Health Committee
LinkOut - more resources
Full Text Sources
Research Materials

