Efficacy and Safety Results With Rilzabrutinib, an Oral Bruton Tyrosine Kinase Inhibitor, in Patients With Immune Thrombocytopenia: Phase 2 Part B Study
- PMID: 39844469
- PMCID: PMC11803537
- DOI: 10.1002/ajh.27539
Efficacy and Safety Results With Rilzabrutinib, an Oral Bruton Tyrosine Kinase Inhibitor, in Patients With Immune Thrombocytopenia: Phase 2 Part B Study
Abstract
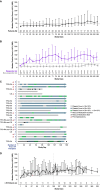
Current treatments for persistent or chronic immune thrombocytopenia (ITP) are limited by inadequate response, toxicity, and impaired quality of life. The Bruton tyrosine kinase inhibitor rilzabrutinib was evaluated to further characterize safety and durability of platelet response. LUNA2 Part B is a multicenter, phase 1/2 study in adults with ITP (≥ 3 months duration, platelet count < 30 × 109/L) who failed ≥ 1 ITP therapy (NCT03395210, EudraCT 2017-004012-19). Oral rilzabrutinib 400 mg bid was given over 24 weeks, with optional long-term extension (LTE). Primary endpoints were safety and platelet counts ≥ 50 × 109/L on ≥ 8 of the last 12 weeks of main treatment without rescue medication. From 22 March2018 to 31 January2023, 26 patients were enrolled. Patients had baseline median platelet count 13 × 109/L, ITP duration 10.3 years, and six prior ITP therapies (46% splenectomized). Nine (35%) patients achieved the primary endpoint. Platelet counts ≥ 50 × 109/L or ≥ 30 × 109/L and doubling from baseline without rescue therapy were sustained for a mean 9.3 weeks. 11 (42%) LTE-eligible patients were ongoing with median LTE platelet > 80 × 109/L. Three (12%) patients received rescue medication during main treatment, none in LTE. Clinically meaningful improvements were observed in fatigue and women's health. With a median treatment duration of 167 days (main treatment), 16 (62%) patients had ≥ 1 treatment-related adverse event (AE), mainly grade 1, including diarrhea (35%), headache (23%), and nausea (15%). There was no treatment-related grade ≥ 2 bleeding/thrombotic events/infections, serious AE, or death. Rilzabrutinib continues to demonstrate durable platelet responses with favorable safety profile in previously treated ITP patients. Trial Registration: NCT03395210, EudraCT 2017-004012-19.
Keywords: adults; immune thrombocytopenia; platelets; quality of life; response.
© 2025 Sanofi and The Author(s). American Journal of Hematology published by Wiley Periodicals LLC.
Conflict of interest statement
Nichola Cooper: reports support for present manuscript from Sanofi; partially supported from the imperial NIHR BRC; research support/grant funding for institution for separate studies from Novartis (ITP) and Rigel (COVID‐19); consulting fees for treatments for ITP from argenyx, Novartis, Sanofi, and Sobi; honoraria for lectures at educational meetings from Novartis, Sanofi, and Sobi; and support for travel and hotel accommodations for ASH 2023 and EHA 2024 from Novartis. A. J. Gerard Jansen: reports grant for ultrasound in hemophilia care from Sobi; Heimburger Award from CSL Behring; consulting fees from Amgen and Novartis; payment or honoraria for lectures, presentations, speakers bureau, manuscript writing or educational events from Amgen, Novartis, and Sobi; support for attending meetings and/or travel from 3SBio, Amgen, Novartis, and Sobi; patent P133951EP00 granted 01‐11‐2022; participation on a data safety monitoring board or advisory board from Novartis; unpaid leadership or fiduciary role in other board, society, committee, or advocacy group as chair NVvH ITP working group, secretary NVvH scientific committee NVB, and Erasmus MC ethics committee; and receipt of drugs for clinical trials from argenx, Principia, and Sanofi. Robert Bird: no financial disclosures to report. Jiří Mayer: support for the present manuscript and grants or contracts from Sanofi. Michelle Sholzberg: reports honoraria from Medison and Sobi. Michael D. Tarantino: consulting fees from Amgen, Biomarin, Dova Pharmaceuticals, Genentech, Novo Nordisk, Octapharma, Pfizer, Sanofi, Sobi‐Swedish Orphan Biovirtum, Takeda, and USB Biosciences; payment or honoraria from Amgen, Biomarin, Genentech, Novartis, Sanofi, and Sobi‐Swedish Orphan Biovirtum; and participation on data safety monitoring board or advisory board for Octapharma and Takeda. Mamta Garg: payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Amgen, AOP Orphan Pharmaceuticals, Janssen J&J, Novartis, and Pfizer; support for attending meetings and/or travel from Amgen, AOP Orphan Pharmaceuticals, Bristol Myers Squibb (Celgene), Janssen J&J, Novartis, Sanofi, and Stemline Therapeutics; and participation on data safety monitoring board or advisory board for Amgen, Bristol Myers Squibb (Celgene), CTI BioPharma, Janssen J&J, and Stemline Therapeutics. Paula F. Ypma: no financial disclosures to report. Vickie McDonald: payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Amgen, Grifols, Novartis, and Sobi; and support for attending meetings and/or travel from Novartis. Charles Percy: no financial disclosures to report. Milan Košťál: no financial disclosures to report. Isaac Goncalves: no financial disclosures to report. Lachezar H. Bogdanov: no financial disclosures to report. Terry B. Gernsheimer: research support for patient enrollment in LUNA phase 2 study; consulting fees from Amgen, Alpine Immune Sciences, argenx, Bioproducts Laboratory (Kedrion), Cellphire, and Sanofi; payment or honoraria for presentation/video from Sanofi; support for travel to advisory board meeting from Novartis (June 2024) and Sanofi (July 2022); and participation on advisory board for Sanofi (July 2022). Remco Diab, Mengjie Yao, and Ahmed Daak report current employment and current equity holders in the publicly held company Sanofi. David J. Kuter: reports research support to institution from Sanofi; consulting from Sanofi; grants or contracts to the institution from BioCryst, HUTCHMED, Novartis, Principia, and Sanofi; royalties from UpToDate; consulting fees from, payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or education events, and receipt of equipment, materials, drugs, medical writing, gifts or other services from Alexion, Alnylam, Alpine, Amgen, Apellis, argenx, BioCryst, Bristol Myers Squibb, Caremark, Cellularity, Cellphire, Chugai, Jiangsu Hengrui, HUTCHMED, Immunovant, Inmagene Bio, Medscape, Merck Sharp & Dohme, Novartis, PER, Pfizer, Platelet Disorder Support Association (PDSA), Principia, Regeneron, Rigel, Sanofi, Seismic, Sobi, Takeda, UpToDate, Verve; and other non‐financial interests with the PDSA medical advisory board.
Figures
References
-
- Adelborg K., Kristensen N. R., Norgaard M., et al., “Cardiovascular and Bleeding Outcomes in a Population‐Based Cohort of Patients With Chronic Immune Thrombocytopenia,” Journal of Thrombosis and Haemostasis 17, no. 6 (2019): 912–924. - PubMed
-
- Cooper N. and Ghanima W., “Immune Thrombocytopenia,” New England Journal of Medicine 381, no. 10 (2019): 945–955. - PubMed
-
- Efficace F., Mandelli F., Fazi P., et al., “Health‐Related Quality of Life and Burden of Fatigue in Patients With Primary Immune Thrombocytopenia by Phase of Disease,” American Journal of Hematology 91, no. 10 (2016): 995–1001. - PubMed
-
- Rodeghiero F., Stasi R., Gernsheimer T., et al., “Standardization of Terminology, Definitions and Outcome Criteria in Immune Thrombocytopenic Purpura of Adults and Children: Report From an International Working Group,” Blood 113, no. 11 (2009): 2386–2393. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

