The splicing auxiliary factor OsU2AF35a enhances thermotolerance via protein separation and promoting proper splicing of OsHSA32 pre-mRNA in rice
- PMID: 39844526
- PMCID: PMC11933845
- DOI: 10.1111/pbi.14587
The splicing auxiliary factor OsU2AF35a enhances thermotolerance via protein separation and promoting proper splicing of OsHSA32 pre-mRNA in rice
Abstract
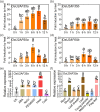
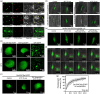
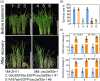
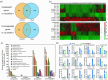
Heat stress significantly impacts global rice production, highlighting the critical need to understand the genetic basis of heat resistance in rice. U2AF (U2 snRNP auxiliary factor) is an essential splicing complex with critical roles in recognizing the 3'-splice site of precursor messenger RNAs (pre-mRNAs). The U2AF small subunit (U2AF35) can bind to the 3'-AG intron border and promote U2 snRNP binding to the branch-point sequences of introns through interaction with the U2AF large subunit (U2AF65). However, the functions of U2AF35 in plants are poorly understood. In this study, we discovered that the OsU2AF35a gene was vigorously induced by heat stress and could positively regulate rice thermotolerance during both the seedling and reproductive growth stages. OsU2AF35a interacts with OsU2AF65a within the nucleus, and both of them can form condensates through liquid-liquid phase separation (LLPS) following heat stress. The intrinsically disordered regions (IDR) are accountable for their LLPS. OsU2AF35a condensation is indispensable for thermotolerance. RNA-seq analysis disclosed that, subsequent to heat treatment, the expression levels of several genes associated with water deficiency and oxidative stress in osu2af35a-1 were markedly lower than those in ZH11. In accordance with this, OsU2AF35a is capable of positively regulating the oxidative stress resistance of rice. The pre-mRNAs of a considerable number of genes in the osu2af35a-1 mutant exhibited defective splicing, among which was the OsHSA32 gene. Knocking out OsHSA32 significantly reduced the thermotolerance of rice, while overexpressing OsHSA32 could partially rescue the heat sensitivity of osu2af35a-1. Together, our findings uncovered the essential role of OsU2AF35a in rice heat stress response through protein separation and regulating alternative pre-mRNA splicing.
Keywords: OsHSA32; OsU2AF35a; condensation; liquid–liquid phase separation; pre‐mRNA splicing; rice thermotolerance.
© 2025 The Author(s). Plant Biotechnology Journal published by Society for Experimental Biology and The Association of Applied Biologists and John Wiley & Sons Ltd.
Conflict of interest statement
None declared.
Figures





References
MeSH terms
Substances
Grants and funding
- 202410389292/College Students Innovation and Entrepreneurship Training Program of Fujian Agriculture and Forestry University
- X202310389245/College Students Innovation and Entrepreneurship Training Program of Fujian Agriculture and Forestry University
- 32171932/National Natural Science Foundation of China
- 2021J01088/Natural Science Foundation of Fujian Province
- 2023J02010/Natural Science Foundation of Fujian Province
LinkOut - more resources
Full Text Sources

