Interaction of B0AT1 Deficiency and Diet on Metabolic Function and Diabetes Incidence in Male Nonobese Diabetic Mice
- PMID: 39844660
- PMCID: PMC11815506
- DOI: 10.1210/endocr/bqaf016
Interaction of B0AT1 Deficiency and Diet on Metabolic Function and Diabetes Incidence in Male Nonobese Diabetic Mice
Abstract
Context: The obesity epidemic parallels an increasing type 1 diabetes incidence, such that westernized diets, containing high fat, sugar, and/or protein, through inducing nutrient-induced islet β-cell stress, have been proposed as contributing factors. The broad-spectrum neutral amino acid transporter (B0AT1), encoded by Slc6a19, is the major neutral amino acids transporter in intestine and kidney. B0AT1 deficiency in C567Bl/6J mice causes aminoaciduria, lowers insulinemia, and improves glucose tolerance.
Objective: We investigated the effects of standard rodent chow (chow), high-fat high-sucrose (HFHS), and high-fat high-protein (HFHP) diets, in addition to B0AT1 deficiency, on the diabetes incidence of male nonobese diabetic (NOD/ShiLtJArc (NOD)) mice.
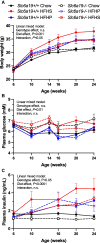
Methods: Male NOD.Slc6a19+/+ and NOD.Slc6a19-/- mice were fed chow, HFHS and HFHP diets from 6 to 24 weeks of age. A separate cohort of male NOD mice were fed the three diets from 6-30 weeks of age. Body weight and fed-state blood glucose and plasma insulin were monitored, and urinary amino-acid profiles, intraperitoneal glucose tolerance, diabetes incidence, pancreatic islet number, insulitis scores and beta-cell mass were measured.
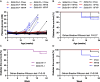
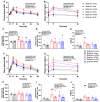
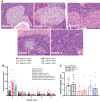
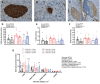
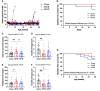
Results: The incidence of diabetes and severe glucose intolerance was 3.8% in HFHS-fed, 25.0% in HFHP-fed, and 14.7% in chow-fed mice, with higher pancreatic islet number and lower insulitis scores in HFHS-fed mice. B0AT1 deficiency had no effect on diabetes incidence, but curtailed HFHS-induced excessive weight gain, adipose tissue expansion, and hyperinsulinemia. In HFHP-fed mice, B0AT1 deficiency significantly increased pancreatic β-cell clusters and small islets. Male NOD mice that did not develop autoimmune diabetes were resistant to diet-induced hyperglycemia.
Conclusion: Dietary composition does, but B0AT1 deficiency does not, affect autoimmune diabetes incidence in male NOD mice. B0AT1 deficiency, however, reduces diet-induced metabolic dysfunction and in HFHP-fed mice increases pancreatic β-cell clusters and small islets.
Keywords: Slc6a19; broad-spectrum neutral amino acid transporter; high-fat high-protein diet; high-fat high-sucrose diet; nonobese diabetic mice; type 1 diabetes.
© The Author(s) 2025. Published by Oxford University Press on behalf of the Endocrine Society.
Figures

References
-
- Gregory GA, Robinson TIG, Linklater SE, et al. Global incidence, prevalence, and mortality of type 1 diabetes in 2021 with projection to 2040: a modelling study. Lancet Diabetes Endocrinol. 2022;10(10):741‐760. - PubMed
-
- Patterson CC, Dahlquist GG, Gyurus E, Green A, Soltesz G; EURODIAB Study Group . Incidence trends for childhood type 1 diabetes in Europe during 1989-2003 and predicted new cases 2005-20: a multicentre prospective registration study. Lancet. 2009;373(9680):2027‐2033. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

