State of precision medicine for heart failure with preserved ejection fraction in a new therapeutic age
- PMID: 39844745
- PMCID: PMC12055434
- DOI: 10.1002/ehf2.15205
State of precision medicine for heart failure with preserved ejection fraction in a new therapeutic age
Abstract
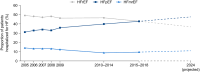
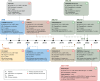
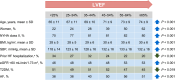
Heart failure with preserved ejection fraction (HFpEF) is defined by heart failure (HF) with a left ventricular ejection fraction (LVEF) of at least 50%. HFpEF has a complex and heterogeneous pathophysiology with multiple co-morbidities contributing to its presentation. Establishing the diagnosis of HFpEF can be challenging. Two algorithms, the 'Heavy, 2 or more Hypertensive drugs, atrial Fibrillation, Pulmonary hypertension, Elderly age >60, elevated Filling pressures' (H2FPEF) and the 'Heart Failure Association Pre-test assessment, Echocardiography and natriuretic peptide, Functional testing, Final aetiology' (HFA-PEFF), can help to determine the likelihood of HFpEF in individuals with symptoms of HF. Phenotype clusters defined largely by the total number and types of co-morbidities may delineate groups of patients with HFpEF with different management needs. It is important to recognize alternative diagnoses or HFpEF mimics such as infiltrative cardiomyopathies, coronary artery disease, lung disease, anxiety, depression, anaemia, severe obesity, and physical deconditioning, among others. Treatment with sodium-glucose co-transporter 2 inhibitors (dapagliflozin and empagliflozin) is recommended for all patients with HFpEF unless contraindicated. Future research should consider alternative approaches to guide the initial diagnosis and treatment of HFpEF, including phenotype clustering models and artificial intelligence, and consider whether LVEF is the most useful distinguishing feature for categorizing HF. Ongoing clinical trials are evaluating novel pharmacological and device-based approaches to address the pathophysiological consequences of HFpEF.
Keywords: Clinical algorithms; Diagnosis; Heart failure with preserved ejection fraction; Management; Phenotypes; Precision medicine; SGLT2i; Sodium‐glucose co‐transporter 2 inhibitors; Treatment.
© 2025 The Author(s). ESC Heart Failure published by John Wiley & Sons Ltd on behalf of European Society of Cardiology.
Conflict of interest statement
Boehringer Ingelheim‐Lilly Alliance was given the opportunity to review the manuscript for medical and scientific accuracy as it relates to Boehringer Ingelheim substances, as well as intellectual property considerations. Boehringer Ingelheim‐Lilly Alliance had no role in the design, analysis, or interpretation of the results in this study. Roy Rasalam reports providing medical education consultancy or has sat on advisory boards for AstraZeneca, Boehringer Ingelheim, Eli Lilly Australia, GSK Australia, MSD Australia, Novo Nordisk, and Sanofi‐Aventis Australia. Andrew Sindone reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, CSL, Edwards, Eli Lilly, GlaxoSmithKline, Healthed, Menarini, Merck Sharp and Dohm, Moderna, Mylan, Novartis, Otsuka Pharmaceutical, Pfizer, Roche, Sanofi, Servier, and Vifor; travel support from Boehringer Ingelheim, CSL, Pharmacosmos, Novartis, and Vifor; participation on advisory boards for AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, CSL, Eli Lilly, GlaxoSmithKline, Menarini, Merck Sharp and Dohm, Moderna, Mylan, Novartis, Otsuka Pharmaceutical, Pfizer, Roche, Sanofi, Servier, and Vifor; is co‐author on the Australia and New Zealand Heart Failure Guidelines, the Australian Heart Failure Consensus Statement, and the Asian and Pacific Heart Failure Consensus Statement; and is co‐chair of the Cardiovascular Expert Reference Group and chair of the Heart Failure Community of Practice for the NSW Agency for Clinical Innovation. Gary Deed reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from AA‐Med, Abbott, AstraZeneca, Boehringer Ingelheim, Healthed, Lilly, Merck Sharp and Dohm, Novartis, Novo Nordisk, and Sanofi; advisory board participation for AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, Merck Sharp and Dohm, Novartis, and Sanofi; was editor of ‘The Royal Australian College of General Practitioners. Management of type 2 diabetes: A handbook of general practice’; is chair of the RACGP Diabetes Specific Interest Group; and is a member of the Australian Diabetes Society Clinical Guidelines/Advisory Committee and the DOHA Chronic Wound Consumables Scheme Committee. Ralph G Audehm reports consulting fees, payment, or honoraria for participation on advisory board for AstraZeneca, Boehringer Ingelheim, Eli Lilly, and NovoNordisk. John J Atherton was a member of the 2023 Australian Therapeutic Guidelines Cardiovascular Expert Group and reports research funding, travel support and/or honoraria paid to his employer for lectures and/or advisory boards for AstraZeneca, Bayer, Boehringer Ingelheim, Bristol‐Myers Squibb, Eli Lilly, Merck Sharp and Dome, Novo Nordisk, Novartis, Roche Diagnostics, and Vifor Pharma.
Figures


References
-
- Atherton JJ, Sindone A, De Pasquale CG, Driscoll A, MacDonald PS, Hopper I, et al. National Heart Foundation of Australia and Cardiac Society of Australia and New Zealand: guidelines for the prevention, detection, and management of heart failure in Australia 2018. Heart Lung Circ 2018;27:1123‐1208. doi:10.1016/j.hlc.2018.06.1042 - DOI - PubMed
-
- Bozkurt B, Coats AJS, Tsutsui H, Abdelhamid CM, Adamopoulos S, Albert N. Universal definition and classification of heart failure: a report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure: Endorsed by the Canadian Heart Failure Society, Heart Failure Association of India, Cardiac Society of Australia and New Zealand, and Chinese Heart Failure Association. Eur J Heart Fail 2021;23:352‐380. doi:10.1002/ejhf.2115 - DOI - PubMed
-
- Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation 2022;145:e895‐e1032. doi:10.1161/CIR.0000000000001063 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

