Morinda officinalis oligosaccharides alleviate chronic unpredictable mild stress-induced depression through the BDNF/TrkB/CREB pathway and symptoms of sexual dysfunction in mice
- PMID: 39844852
- PMCID: PMC11750790
- DOI: 10.3389/fnins.2024.1509543
Morinda officinalis oligosaccharides alleviate chronic unpredictable mild stress-induced depression through the BDNF/TrkB/CREB pathway and symptoms of sexual dysfunction in mice
Abstract
Background: In recent years, depression has become a global public health concern, and one of the common concomitant symptoms are diminished sexual motivation and impaired sexual performance. The aim of this study was to investigate the potential effects of Morinda officinalis oligosaccharides (MOO) on depression and its concomitant symptom, sexual dysfunction.
Methods: Chronic unpredictable mild stress (CUMS)-induced depression model was constructed, and the effects of MOO on depression and sexual abilities were evaluated.
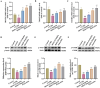
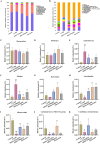
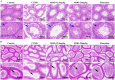
Results: The results revealed that MOO was able to alleviate CUMS-induced depression-like behavior in mice, to inhibit hippocampal neuron apoptosis, to reverse monoamine neurotransmitter imbalance, increase Brain-derived neurotrophic factor (BDNF) expression levels in the hippocampus, to modulate the composition and distribution of gut microbiota, and to increase the abundance of probiotics after continuous gavage of MOO for 28 days. MOO further confirmed that sexual dysfunction is closely related to the development of depression by improving the lack of sexual motivation and low sexual performance in CUMS-induced depressed mice, modulating the disruption of sex hormone secretion in serum, and alleviating sperm morphology and functional defects in the epididymis.
Conclusion: These findings on MOO provide a basis for exploring its antidepressant mechanism, its use to improve hypogonadotropic symptoms, and for future development of new antidepressant drug to improves hypogonadotropic symptoms.
Keywords: BDNF/TrkB/CREB pathway; CUMS; Morinda officinalis oligosaccharides; depression; gut microbiota; sexual dysfunction.
Copyright © 2025 He, Hu, Wang, Zuo, Li, Zhao, Hao, Dai, Wang and Sun.
Conflict of interest statement
ZZu, HL, and ZZh were employed by Beijing Tongrentang Company Limited. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Buchenauer L., Haange S., Bauer M., Rolle-Kampczyk U., Wagner M., Stucke J., et al. (2023). Maternal exposure of mice to glyphosate induces depression- and anxiety-like behavior in the offspring via alterations of the gut-brain axis. Sci. Total Environ. 905:167034. doi: 10.1016/j.scitotenv.2023.167034, PMID: - DOI - PubMed
LinkOut - more resources
Full Text Sources

