Utility of serum biomarkers in real-world practice for predicting response to omalizumab therapy in patients with chronic spontaneous urticaria
- PMID: 39844914
- PMCID: PMC11751516
- DOI: 10.1016/j.jacig.2024.100386
Utility of serum biomarkers in real-world practice for predicting response to omalizumab therapy in patients with chronic spontaneous urticaria
Abstract
Background: Omalizumab (OMA), a recombinant humanized IgG monoclonal anti-IgE antibody, is approved for treatment for chronic spontaneous urticaria (CSU) refractory to second-generation H1-antihistamine (SGAH) therapy. However, currently, there are no validated serum biomarkers to reliably predict response to OMA treatment.
Objective: We explored the real-world clinical utility of using serum biomarkers for predicting response to OMA for CSU patients with disease refractory to high-dose SGAH therapy.
Methods: A single-center, retrospective chart review of CSU patients treated with OMA enrolled patients who had at their initial evaluation collection of a basophil histamine release assay for detecting IgG antibodies targeting FcεR1α subunit before starting OMA treatment. In addition, total IgE, IgG-anti-thyroid peroxidase (TPO), C-reactive protein, and absolute eosinophil count, if available, were analyzed as predictors for OMA response. The validated Outcome and Assessment Information Set Database (OASIS-D) rating system was used to assess responsiveness to OMA.
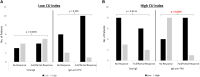
Results: High levels of IgG-anti-TPO were significantly associated with a poor response to OMA. However, basophil histamine release assay, total IgE, C-reactive protein, and absolute eosinophil count, as well as IgG-anti-TPO/total IgE ratios, were not predictive of a response to OMA therapy.
Conclusions: This real-world study confirms previous reports that a high IgG-anti-TPO level is a reliable predictor of poor response to OMA. However, better validation of basophil histamine release assay and other immunoassays that measure IgG antibodies to FcεR1α subunit are required before they can be recommended as predictors for OMA response. Whether any of these biomarkers are relevant for predicting response to novel advanced therapeutics under current development requires further investigation.
Keywords: CU Index; Chronic spontaneous urticaria; IgE; TPO; autoimmune urticaria; biomarkers; omalizumab.
© 2024 The Authors.
Conflict of interest statement
Supported by the Division of Rheumatology, Allergy and Immunology, 10.13039/100008102University of Cincinnati, as part of its allergy training program. Disclosure of potential conflict of interest: J. A. Bernstein is a PI and consultant for Novartis, Genentech, Sanofi, Regeneron, AstraZeneca, Allakos, Celldex, Jasper, Escient, Amgen, Takeda/Shire, Astria, Pharming, CSL Behring, IONIS, Intelia, BioMarin, KalVista, BioCryst, Blueprint Medicine, Cogent, and Telios. The rest of the authors declare that they have no relevant conflicts of interest.
Figures

References
-
- Zuberbier T., Bernstein J.A., Maurer M. Chronic spontaneous urticaria guidelines: what is new? J Allergy Clin Immunol. 2022;150:1249–1255. - PubMed
-
- Bernstein J.A., Lang D.M., Khan D.A., Craig T., Dreyfus D., Hsieh F., et al. The diagnosis and management of acute and chronic urticaria: 2014 update. J Allergy Clin Immunol. 2014;133:1270–1277. - PubMed
-
- Kaplan A., Lebwohl M., Gimenez-Arnau A.M., Hide M., Armstrong A.W., Maurer M. Chronic spontaneous urticaria: focus on pathophysiology to unlock treatment advances. Allergy. 2023;78:389–401. - PubMed
-
- Gimenez-Arnau A.M., DeMontojoye L., Asero R., Cugno M., Kulthanan K., Yanase Y., et al. The pathogenesis of chronic spontaneous urticaria: the role of infiltrating cells. J Allergy Clin Immunol Pract. 2021;9:2195–2208. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

