Hydrothermal carbonization synthesis of amorphous carbon nanoparticles (15-150 nm) with fine-tuning of the size, bulk order, and the consequent impact on antioxidant and photothermal properties
- PMID: 39845136
- PMCID: PMC11748258
- DOI: 10.1039/d4na00923a
Hydrothermal carbonization synthesis of amorphous carbon nanoparticles (15-150 nm) with fine-tuning of the size, bulk order, and the consequent impact on antioxidant and photothermal properties
Abstract
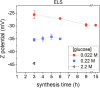
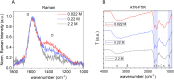
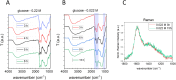
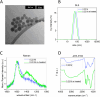
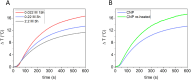
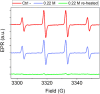
Hydrothermal carbonization (HTC) of carbohydrates has been reported as a sustainable and green technique to produce carbonaceous micro- and nano-materials. These materials have been developed for several applications, including catalysis, separation science, metal ion adsorption and nanomedicine. Carbon nanoparticles (CNPs) obtained through HTC are particularly interesting for the latter application since they exhibit photothermal properties when irradiated with near-infrared (NIR) light, act as an antioxidant by scavenging reactive oxygen species (ROS), and present good colloidal stability and biocompatibility. However, due to the highly disordered structure, there is still a poor understanding of the mechanism of synthesis of CNPs. Consequently, the modulation of the CNP properties by controlling the synthetic parameters is still a challenge. In this work, a novel and simplified HTC synthetic strategy to obtain non-aggregated glucose derived CNPs in the 15-150 nm size range with precise control of the diameter is presented, together with an advance in the understanding of the reaction mechanism behind the synthesis. Modifications of the synthetic parameters and a post-synthesis hydrothermal process were applied to increase the bulk order of CNPs, resulting in an increase of the photothermal and ROS scavenging activities, without affecting the morphological and colloidal properties of the nanomaterial.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Baughman R. H. Zakhidov A. A. De Heer W. A. Carbon nanotubes – the route toward applications. Science. 2002;297:787–792. doi: 10.1126/science.1060928. https://dx.doi.org/10.1126/SCIENCE.1060928 - DOI - DOI - PubMed
-
- Geim A. K. Graphene: status and prospects. Science. 2009;324:1530–1534. doi: 10.1126/science.1158877. https://dx.doi.org/10.1126/SCIENCE.1158877 - DOI - DOI - PubMed
-
- Tian Z. Zhang X. Li D. Zhou D. Jing P. Shen D. Qu S. Zboril R. Rogach A. L. Tian Z. et al., Full-color inorganic carbon dot phosphors for white-light-emitting diodes. Adv. Opt. Mater. 2017;5(19):1700416. doi: 10.1002/adom.201700416. https://dx.doi.org/10.1002/adom.201700416 - DOI - DOI
-
- Picollo F. Mino L. Battiato A. Ditalia Tchernij S. Forneris J. Martina K. Sacco M. Tagliapietra S. Vittone E. Olivero P. et al., Synthesis and characterization of porphyrin functionalized nanodiamonds. Diamond Relat. Mater. 2019;91:22–28. doi: 10.1016/j.diamond.2018.11.001. https://dx.doi.org/10.1016/J.DIAMOND.2018.11.001 - DOI - DOI
-
- Wang M.-J., Gray C. A., Reznek S. A., Mahmud K., Kutsovsky Y., Carbon black, in Kirk-Othmer Encyclopedia of Chemical Technology, 2003, 10.1002/0471238961.0301180204011414.A01.PUB2 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

