Exploring the Cardioprotective Mechanisms of Ligusticum wallichii in Myocardial Infarction Through Network Pharmacology and Experimental Validation
- PMID: 39845152
- PMCID: PMC11750949
- DOI: 10.2147/DDDT.S481499
Exploring the Cardioprotective Mechanisms of Ligusticum wallichii in Myocardial Infarction Through Network Pharmacology and Experimental Validation
Abstract
Background: Myocardial infarction represents a coronary artery ailment with the highest incidence and fatality rates among cardiovascular conditions. However, effective pharmacological interventions remain elusive. This study seeks to elucidate the molecular mechanisms underlying the effects of Ligusticum wallichii on myocardial infarction through network pharmacology and experimental validation.
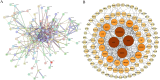
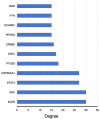
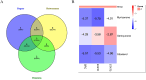
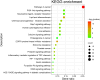
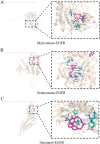
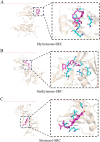
Methods: Initially, potential targets of Ligusticum wallichii's active ingredients and myocardial infarction-related targets were retrieved from databases. Subsequently, core targets of Ligusticum wallichii on myocardial infarction were identified via the PPI network analysis and subjected to GO and KEGG pathway enrichment analyses. Molecular docking was employed to validate the binding affinities between the core targets and the bioactive components. The findings from network pharmacology analysis were corroborated through in vitro and in vivo experiments.
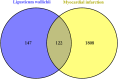
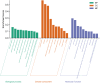
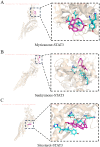
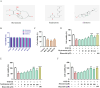
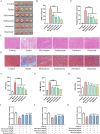
Results: Seven active ingredients from Ligusticum wallichii were identified, corresponding to 122 targets. Molecular docking revealed robust binding affinities of Myricanone, Senkyunone, and Sitosterol to key target proteins (EGFR, STAT3, and SRC). In vitro, experiments demonstrated that pretreatment with the active components of Ligusticum wallichii protected myocardial cells from OGD exposure and modulated the expression of their key target genes. In vivo, experiments showed that the active components of Ligusticum wallichii significantly improved myocardial infarction via alleviating myocardial fibrosis and oxidative stress and did not elicit toxic effects in mice.
Conclusion: The collective findings suggest that Ligusticum wallichii shows promising potential for myocardial infarction treatment by regulating key target proteins (EGFR, STAT3, and SRC), which play roles in oxidative stress and myocardial fibrosis.
Keywords: Ligusticum wallichii; Molecular docking; experimental validation; myocardial infarction; network pharmacology.
© 2025 Yang et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

