Antituberculosis Therapy-Induced Acute Liver Failure in a Renal Transplant Recipient: A Case Report
- PMID: 39845251
- PMCID: PMC11753582
- DOI: 10.7759/cureus.76263
Antituberculosis Therapy-Induced Acute Liver Failure in a Renal Transplant Recipient: A Case Report
Abstract
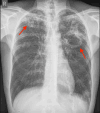
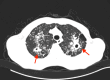
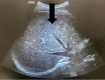
To prevent organ rejection, renal transplant (RT) recipients must take immunosuppressive medicines, which make them more susceptible to infections such as tuberculosis (TB). Hepatotoxicity, which can vary from asymptomatic increased liver enzymes to severe liver failure, is the most prevalent side effect of first-line antituberculosis (AT) drugs. Treating TB in RT patients involves unique concerns since AT medications might interact with immunosuppressive medications, potentially reducing efficacy or increasing toxicity. A 65-year-old RT recipient was diagnosed with active pulmonary TB 18 years after renal transplantation. He had drug-induced acute liver failure after initiating AT therapy, but his liver function improved after discontinuing AT medications and receiving supportive care.
Keywords: : tuberculosis; acute liver failure (alf); drug-induced acute liver failure; drug-induced hepatotoxicity (dih); renal transplant recipient.
Copyright © 2024, Gajić et al.
Conflict of interest statement
Human subjects: Consent for treatment and open access publication was obtained or waived by all participants in this study. Ethics Committee of the Faculty of Medicine, University of Belgrade issued approval Not Applicable. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures

Similar articles
-
API TB Consensus Guidelines 2006: Management of pulmonary tuberculosis, extra-pulmonary tuberculosis and tuberculosis in special situations.J Assoc Physicians India. 2006 Mar;54:219-34. J Assoc Physicians India. 2006. PMID: 16800350
-
Incidence of antituberculosis-drug-induced hepatotoxicity and associated risk factors among tuberculosis patients in Dawro Zone, South Ethiopia: A cohort study.Int J Mycobacteriol. 2016 Mar;5(1):14-20. doi: 10.1016/j.ijmyco.2015.10.002. Epub 2015 Oct 30. Int J Mycobacteriol. 2016. PMID: 26927985
-
Understanding antituberculosis drug-induced hepatotoxicity: Risk factors and effective management strategies in the pediatric population.World J Clin Pediatr. 2025 Jun 9;14(2):101875. doi: 10.5409/wjcp.v14.i2.101875. eCollection 2025 Jun 9. World J Clin Pediatr. 2025. PMID: 40491731 Free PMC article. Review.
-
Anti-tuberculosis drug-induced hepatitis in renal transplant patient with pulmonary and extra pulmonary tuberculosis.Saudi Pharm J. 2012 Apr;20(2):181-5. doi: 10.1016/j.jsps.2011.09.003. Epub 2011 Sep 23. Saudi Pharm J. 2012. PMID: 23960791 Free PMC article.
-
A practical approach to tuberculosis diagnosis and treatment in liver transplant recipients in a low-prevalence area.Med Mal Infect. 2019 Jun;49(4):231-240. doi: 10.1016/j.medmal.2018.11.013. Epub 2018 Dec 24. Med Mal Infect. 2019. PMID: 30591271 Review.
References
-
- Infection in kidney transplantation. Nambiar P, Silibovsky R, Belden KA. Contemp Kidney Transplant. 2018:307–327.
-
- Latent tuberculosis infection and renal transplantation - diagnosis and management. Krishnamoorthy S, Kumaresan N, Zumla A. Int J Infect Dis. 2019;80S:0–6. - PubMed
-
- An official ATS statement: hepatotoxicity of antituberculosis therapy. Saukkonen JJ, Cohn DL, Jasmer RM, et al. Am J Respir Crit Care Med. 2006;174:935–952. - PubMed
-
- Prevention and treatment of tuberculosis in solid organ transplant recipients. Abad CL, Razonable RR. Expert Rev Anti Infect Ther. 2020;18:63–73. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
