Challenges in diagnosing polyethylene glycol and polysorbate 80 allergies: implications for allergic reactions in COVID-19 mRNA vaccination program: experience from Qatar
- PMID: 39845650
- PMCID: PMC11753240
- DOI: 10.3389/falgy.2024.1502285
Challenges in diagnosing polyethylene glycol and polysorbate 80 allergies: implications for allergic reactions in COVID-19 mRNA vaccination program: experience from Qatar
Abstract
Introduction: COVID-19 vaccination has been a key intervention in reducing the severity of symptoms; however, concerns about vaccine safety, particularly regarding allergic reactions, arose early on. Healthcare workers faced the challenge of addressing these concerns to ensure safe vaccine administration. This study aimed to review the practical aspects of using allergy skin testing for COVID-19 vaccine excipients in patients with a history of allergic reactions developed following mRNA COVID-19 vaccination.
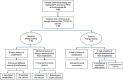
Methods: A retrospective chart review was conducted for patients who reported allergic reactions after the COVID-19 vaccine and underwent allergy skin testing for COVID-19 vaccine excipients in the Adult Allergy and Immunology Service at Hamad Medical Corporation, Doha, Qatar. The testing protocol, developed based on published data during the pandemic, included skin prick (SPT) and intradermal (ID) testing using medications containing polysorbate 80 and polyethylene glycol (PEG), the primary excipients in the COVID-19 vaccines suspected of triggering allergic responses.
Results: Of the 88 patients reviewed, 38 reported different types of allergic reactions following mRNA COVID-19 vaccination, with the majority being female. Anaphylaxis was reported in 21.1% of the patients, while the remaining experienced less severe allergic reactions. All patients underwent SPT and ID testing with PEG and polysorbate 80. By SPT, two patients tested positive for PEG and none for polysorbate 80. By ID, seven tested positive for polysorbate 80 and one for PEG. Among patients who experienced anaphylaxis, 50% had positive allergy test results. Twenty-three percent of patients with negative test results could receive additional vaccine doses without adverse reactions.
Conclusion: Managing patients with a history of allergic reactions to the COVID-19 vaccine is challenging, as the exact mechanisms and accurate and valid allergy testing are yet to be determined. In our cohort, most patients had mild allergic reactions following vaccination. Excipients' allergy skin testing has helped to reduce vaccine hesitancy despite its questionable utility in clinical practice.
Keywords: COVID-19 vaccines; Qatar; allergy testing; anaphylaxis; excipients; polyethylene glycol allergy; polysorbate allergy.
© 2025 Aqel, Thalappil, Imameldin, Mudawi, Al Maslamani, Al-Khal, Mobayed, Al-Nesf and Ibrahim.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Dadras O, SeyedAlinaghi S, Karimi A, Shamsabadi A, Mahdiabadi S, Mohammadi P, et al. Public acceptability of COVID-19 vaccines and its predictors in Middle Eastern/North African (MENA) countries: a systematic review. Hum Vaccines Immunother. (2022) 18(5):2043719. 10.1080/21645515.2022.2043719 - DOI - PMC - PubMed
-
- Hatmal MM, Al-Hatamleh MAI, Olaimat AN, Hatmal M, Alhaj-Qasem DM, Olaimat TM, et al. Side effects and perceptions following COVID-19 vaccination in Jordan: a randomized, cross-sectional study implementing machine learning for predicting severity of side effects. Vaccines. (2021) 9(6):556. 10.3390/vaccines9060556 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources