Evolution of parasite transmission dispersion
- PMID: 39845710
- PMCID: PMC11750388
- DOI: 10.1098/rsos.240629
Evolution of parasite transmission dispersion
Abstract
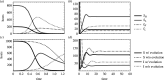
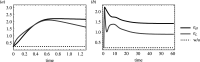
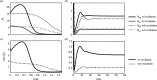
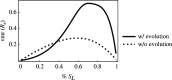
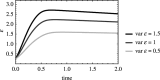
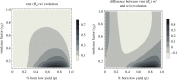
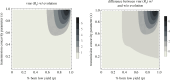
An open question in epidemiology is why transmission is often overdispersed, meaning that most new infections are driven by few infected individuals. For example, around 10% of COVID-19 cases cause 80% of new COVID-19 cases. This overdispersion in parasite transmission is likely driven by intrinsic heterogeneity among hosts, i.e. variable SARS-CoV-2 viral loads. However, host heterogeneity could also indirectly increase transmission dispersion by driving parasite adaptation. Specifically, transmission variation among hosts could drive parasite specialization to highly infectious hosts. Adaptation to rare, highly infectious hosts could amplify transmission dispersion by simultaneously decreasing transmission from common, less infectious hosts. This study considers whether increased transmission dispersion can be, in part, an emergent property of parasite adaptation to heterogeneous host populations. We develop a mathematical model using a Price equation framework to address this question that follows the epidemiological and evolutionary dynamics of a general host-parasite system. The results predict that parasite adaptation to heterogeneous host populations drives high transmission dispersion early in epidemics. Furthermore, parasite adaptation can maintain increased transmission dispersion at endemic equilibria if virulence differs between hosts in a heterogeneous population. More broadly, this study provides a framework for predicting how parasite adaptation determines transmission dispersion for emerging and re-emerging infectious diseases.
Keywords: epidemiology; evolution; parasite; superspreading.
© 2025 The Authors.
Conflict of interest statement
We declare we have no competing interests.
Figures

![The modelling framework follows the epidemiological dynamics of the host population (using a SI model [22]) and the evolutionary dynamics of the parasite within-host parasite growth rate (using the Price equation (19))](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/a5d7/11750388/2ca3c4f4c2b2/rsos.240629.f002.gif)







References
-
- VanderWaal KL, Ezenwa VO. 2016. Heterogeneity in pathogen transmission: mechanisms and methodology. Funct. Ecol. 30, 1606–1622. (10.1111/1365-2435.12645) - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous
