The in vivo metabolic pathway of Deg-AZM and in vitro investigations into the contribution of drug metabolizing enzymes and drug transporters in the drug interactions of Deg-AZM, a clinical-stage new transgelin agonist
- PMID: 39845780
- PMCID: PMC11750672
- DOI: 10.3389/fphar.2024.1510903
The in vivo metabolic pathway of Deg-AZM and in vitro investigations into the contribution of drug metabolizing enzymes and drug transporters in the drug interactions of Deg-AZM, a clinical-stage new transgelin agonist
Abstract
Introduction: Deglycosylated azithromycin (Deg-AZM), a new transgelin agonist with positive therapeutic effects on slow transit constipation, has been approved for clinical trials in 2024. This work investigated the drug metabolism and transport of Deg-AZM to provide research data for further development of Deg-AZM.
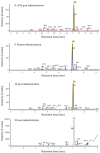
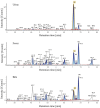
Methods: A combination of UPLC-QTOF-MS was used to obtain metabolite spectra of Deg-AZM in plasma, urine, feces and bile. Caco-2 cells was used to investigate the permeability of Deg-AZM and whether it is a potential substrate of the efflux transporter P-glycoprotein. Human liver microsome phenotyping assays with chemical inhibition and recombinant CYPs phenotyping assays were used to investigate the CYP450 enzyme phenotype involved in Deg-AZM metabolism in vitro. A HLM inhibition reaction system was established to evaluate the inhibitory effect of Deg-AZM on CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, and CYP3A4. The mRNA expression of human primary hepatocytes incubated with Deg-AZM or not was evaluate the induction of Deg-AZM on CYP1A2, CYP2B6, and CYP3A4.
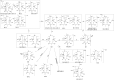
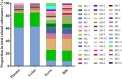
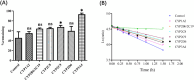
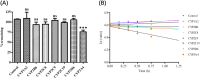
Results: 44 metabolites of Deg-AZM were identified in rat urine, feces, bile, and plasma, the metabolic pathways included demethylation, monohydroxylation, dihydroxylation, dehydroxidation, hydroreduction, hydrolysis, methylation, glucuronidation and the combination of different metabolic pathways. Deg-AZM was a low permeability drug in the intestine and a potential substrate of the efflux transporter P-glycoprotein. CYP3A4 was the major CYP isoform responsible for Deg-AZM metabolism. Deg-AZM showed moderate inhibition with CYP2B6 and CYP2D6. Data in three batches of human primary hepatocytes disclosed induction potential of Deg-AZM on CYP2B6 and CYP3A4.
Conclusion: The in vivo metabolic pathway of Deg-AZM and in vitro possibility of drug interaction for Deg-AZM with CYP enzymes and drug transporter were fully investigated. It was suggested that dose adjustments may be warranted depending on the potency of the corresponding modulators in clinical.
Keywords: CYP enzymes; Caco-2 cell; P-gp; deglycosylated azithromycin; drug-drug interactions; metabolic pathways.
Copyright © 2025 Gu, Li, Tian, Zheng, Cai, Xu, Zhao, Liu, Sun, Luo, Zhu, Zhou, Ai and Yang.
Conflict of interest statement
Author WT was employed by The National Institutes of Pharmaceutical R&D Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources

