Engineering perfluoroarenes for enhanced molecular barrier effect and chirality transfer in solutions
- PMID: 39845871
- PMCID: PMC11750069
- DOI: 10.1039/d4sc07859d
Engineering perfluoroarenes for enhanced molecular barrier effect and chirality transfer in solutions
Abstract
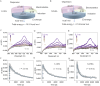
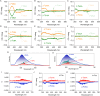
Noncovalent forces have a significant impact on photophysical properties, and the flexible employment of weak forces facilitates the design of novel luminescent materials with a variety of applications. The arene-perfluoroarene (AP) force, as one type of π-hole/π interaction, shows unique directionality, involving an electron-deficient π-hole interacting with a π-electron-rich region, facilitating precise orientation and stabilization in supramolecular structures. Here we present an amination engineering protocol to build a perfluoroarene library based on an octafluoronaphthalene skeleton with various steric and electronic properties. In diluted solution-based assemblies, the perfluoroarenes perform as efficient molecular barriers to perylene building units, lighting up the luminescence. Enhanced steric effects, hydrophobicity and appended aromatic pendants are pivotal structural factors to boost the molecular barrier effect. Highly affinitive AP coassemblies transfer chirality from perfluoroarenes to achiral perylene moieties, inducing the appearance of chiral microstructures with tailored circularly polarized luminescence. Application as luminescent ink for enhanced water-resistance in displays and anti-counterfeiting is successfully realized. This work greatly extends the potential of molecular engineering in noncovalently bonded luminescent materials, and clearly reveals structure-property correlations.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures






Similar articles
-
Hierarchically Evolved Supramolecular Chirality Mediated by Arene-Perfluoroarene Interaction.ACS Appl Mater Interfaces. 2021 Jun 23;13(24):29170-29178. doi: 10.1021/acsami.1c07720. Epub 2021 Jun 9. ACS Appl Mater Interfaces. 2021. PMID: 34105347
-
Improving the Overall Properties of Circularly Polarized Luminescent Materials Through Arene-Perfluoroarene Interactions.Angew Chem Int Ed Engl. 2021 Feb 23;60(9):4575-4580. doi: 10.1002/anie.202014891. Epub 2021 Jan 7. Angew Chem Int Ed Engl. 2021. PMID: 33236479
-
Arene-Perfluoroarene Force Driven Sublimation-Removable Chiral Coassemblies.Angew Chem Int Ed Engl. 2023 Jun 19;62(25):e202305135. doi: 10.1002/anie.202305135. Epub 2023 May 9. Angew Chem Int Ed Engl. 2023. PMID: 37092858
-
Circularly Polarized Luminescence in Nanoassemblies: Generation, Amplification, and Application.Adv Mater. 2020 Oct;32(41):e1900110. doi: 10.1002/adma.201900110. Epub 2019 Aug 8. Adv Mater. 2020. PMID: 31394014 Review.
-
Principles of Cation-π Interactions for Engineering Mussel-Inspired Functional Materials.Acc Chem Res. 2022 Apr 19;55(8):1171-1182. doi: 10.1021/acs.accounts.2c00068. Epub 2022 Mar 28. Acc Chem Res. 2022. PMID: 35344662 Review.
References
-
- Kou X. Xu X. Gao N. Zhang Y. Huang X. Chen F. Ke Q. Meng Q. Food Hydrocolloids. 2024;157:110441. doi: 10.1016/j.foodhyd.2024.110441. - DOI
-
- Liu Z. Dai X. Sun Y. Liu Y. Aggregate. 2020;1:31. doi: 10.1002/agt2.3. - DOI
-
- Xu L. Miao X. Ying X. Deng W. J. Phys. Chem., C. 2011;116:1061. doi: 10.1021/jp210000e. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

