Molecular profiling of frontal and occipital subcortical white matter hyperintensities in Alzheimer's disease
- PMID: 39845935
- PMCID: PMC11753232
- DOI: 10.3389/fneur.2024.1470441
Molecular profiling of frontal and occipital subcortical white matter hyperintensities in Alzheimer's disease
Abstract
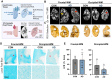
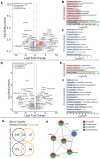
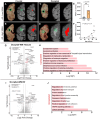
White matter hyperintensities (WMHs) are commonly detected on T2-weighted magnetic resonance imaging (MRI) scans, occurring in both typical aging and Alzheimer's disease (AD). Despite their frequent appearance and their association with cognitive decline in AD, the molecular factors contributing to WMHs remain unclear. In this study, we investigated the transcriptomic profiles of two commonly affected brain regions with coincident AD pathology-frontal subcortical white matter (frontal-WM) and occipital subcortical white matter (occipital-WM)-and compared with age-matched cognitively intact controls. Through RNA-sequencing in frontal- and occipital-WM bulk tissues, we identified an upregulation of genes associated with brain vasculature function in AD white matter. To further elucidate vasculature-specific transcriptomic features, we performed RNA-seq analysis on blood vessels isolated from these white matter regions, which revealed an upregulation of genes related to protein folding pathways. Finally, comparing gene expression profiles between AD individuals with high- versus low-WMH burden showed an increased expression of pathways associated with immune function. Taken together, our study characterizes the diverse molecular profiles of white matter changes in AD and provides mechanistic insights into the processes underlying AD-related WMHs.
Keywords: Alzheimer’s disease; angiogenesis; blood vessels; brain vasculature; heat shock proteins (HSPs); protein folding; white matter hyperintensities (WMHs).
Copyright © 2025 Malla, Bryant, Jayakumar, Woost, Wolf, Li, Das, van Veluw and Bennett.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures


Update of
-
Molecular profiling of frontal and occipital subcortical white matter hyperintensities in Alzheimer's disease.bioRxiv [Preprint]. 2024 Jun 13:2024.06.13.598845. doi: 10.1101/2024.06.13.598845. bioRxiv. 2024. Update in: Front Neurol. 2025 Jan 07;15:1470441. doi: 10.3389/fneur.2024.1470441. PMID: 38915516 Free PMC article. Updated. Preprint.
Similar articles
-
Molecular profiling of frontal and occipital subcortical white matter hyperintensities in Alzheimer's disease.bioRxiv [Preprint]. 2024 Jun 13:2024.06.13.598845. doi: 10.1101/2024.06.13.598845. bioRxiv. 2024. Update in: Front Neurol. 2025 Jan 07;15:1470441. doi: 10.3389/fneur.2024.1470441. PMID: 38915516 Free PMC article. Updated. Preprint.
-
White matter hyperintensities and TDP-43 pathology in Alzheimer's disease.Alzheimers Dement. 2025 Feb;21(2):ealz14516. doi: 10.1002/alz.14516. Epub 2025 Jan 17. Alzheimers Dement. 2025. PMID: 39821594 Free PMC article.
-
Characterization of white matter hyperintensities in Down syndrome.Alzheimers Dement. 2024 Sep;20(9):6527-6541. doi: 10.1002/alz.14146. Epub 2024 Aug 1. Alzheimers Dement. 2024. PMID: 39087352 Free PMC article.
-
White matter hyperintensities are higher among early-onset Alzheimer's disease participants than their cognitively normal and early-onset nonAD peers: Longitudinal Early-onset Alzheimer's Disease Study (LEADS).Alzheimers Dement. 2023 Nov;19 Suppl 9(Suppl 9):S89-S97. doi: 10.1002/alz.13402. Epub 2023 Jul 25. Alzheimers Dement. 2023. PMID: 37491599 Free PMC article. Review.
-
White matter hyperintensities in vascular contributions to cognitive impairment and dementia (VCID): Knowledge gaps and opportunities.Alzheimers Dement (N Y). 2019 Apr 9;5:107-117. doi: 10.1016/j.trci.2019.02.001. eCollection 2019. Alzheimers Dement (N Y). 2019. PMID: 31011621 Free PMC article. Review.
References
-
- de Leeuw FE, de Groot JC, Achten E, Oudkerk M, Ramos LM, Heijboer R, et al. . Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. The Rotterdam scan study. J Neurol Neurosurg Psychiatry. (2001) 70:9–14. doi: 10.1136/jnnp.70.1.9, PMID: - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

