Anti-Inflammatory and Antioxidant Effects of Gundelia tournefortii Aqueous Extract on the Liver and Kidney of PCOS Mice
- PMID: 39846229
- PMCID: PMC11755546
- DOI: 10.1177/2515690X241304519
Anti-Inflammatory and Antioxidant Effects of Gundelia tournefortii Aqueous Extract on the Liver and Kidney of PCOS Mice
Abstract
Background: Polycystic Ovarian Syndrome (PCOS) is an endocrine disorder associated with increased risk of kidney and liver damage. Current treatments have shown contradictory outcomes, and their long-term use causes unwanted side effects. G. tournefortii could serve as a complementary medicine to current PCOS treatments.
Purpose: This study evaluates the effect of G. tournefortii in alleviating liver and kidney damage induced by PCOS via the regulation of oxidative stress pathways.
Study design: PCOS was induced in female Balb/c mice using dehydroepiandrosterone over 21 days. They included a Sham group, a Vehicle group, a group treated with the extract only, and an untreated PCOS mice group. Positive Controls were treated with Metformin. The other PCOS groups were either co-treated while inducing PCOS or treated with the extract post-disease induction.
Methods: Histological analysis was performed. Serum liver and kidney biochemical markers, levels of oxidative stress, and two pro-inflammatory markers were measured. NLRP3 and its associated genes (caspase-1 and ASC) gene expression was assessed.
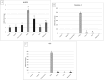
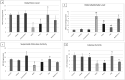
Results: The extract restored normal kidney and liver histology post-PCOS induction. It decreased ALT and AST levels by 50% and the oxidant marker malondialdehyde (MDA) by 65% (P < .05). Superoxide dismutase (SOD)/catalase (CAT) activities were normalized in PCOS treated group. IL-1β/TNF-α significantly decreased (80% and 68%, respectively, P < .05) in the post-treated group. NLRP3 genes decreased in kidney tissues post-treatment with G. tournefortii extract.
Conclusion: G. tournefortii reduced oxidative stress by modifying the ASC/caspase-1/IL-1β signaling pathway, thus protecting livers and kidneys highlighting the herb as a potential preventative and complementary agent in mitigating PCOS associated damage.
Keywords: DHEA-induced mice; Gundelia tournefortii; kidney injury; liver injury; polycystic ovarian syndrome; polyphenols.
Conflict of interest statement
Declaration of Conflicting InterestsThe authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures








Similar articles
-
Inhibition of oxidative stress and NLRP3 inflammasome by Saikosaponin-d alleviates acute liver injury in carbon tetrachloride-induced hepatitis in mice.Int J Immunopathol Pharmacol. 2020 Jan-Dec;34:2058738420950593. doi: 10.1177/2058738420950593. Int J Immunopathol Pharmacol. 2020. PMID: 32816567 Free PMC article.
-
Protective Potential of Methanol Extract of Drymaria cordata Willd. ex Schult (MEDC) on Letrozole-Induced Polycystic Ovary Syndrome Via Modulation of Apoptotic Markers, Sex Hormones and Antioxidant Status in Rat Model.Reprod Sci. 2024 Sep;31(9):2849-2860. doi: 10.1007/s43032-024-01597-6. Epub 2024 May 21. Reprod Sci. 2024. PMID: 38773025
-
The ameliorative effects of marjoram in dehydroepiandrosterone induced polycystic ovary syndrome in rats.Life Sci. 2020 Nov 15;261:118353. doi: 10.1016/j.lfs.2020.118353. Epub 2020 Aug 30. Life Sci. 2020. PMID: 32877649
-
A comprehensive insight into effects of green tea extract in polycystic ovary syndrome: a systematic review.Reprod Biol Endocrinol. 2021 Sep 23;19(1):147. doi: 10.1186/s12958-021-00831-z. Reprod Biol Endocrinol. 2021. PMID: 34551795 Free PMC article.
-
Anti-infertility roles of flavonoids: insights into the female reproductive system.Mol Biol Rep. 2025 May 22;52(1):495. doi: 10.1007/s11033-025-10579-z. Mol Biol Rep. 2025. PMID: 40404937 Review.
References
-
- Escobar-Morreale HF. Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol. 2018;14(5):270-284. doi:10.1038/nrendo.2018.24. Pubmed link: https://pubmed.ncbi.nlm.nih.gov/29569621/ - DOI - PubMed
-
- Azziz R. Polycystic ovary syndrome. Obstet Gynecol. 2018;132(2):321-336. doi:10.1097/aog.0000000000002698. Pubmed link: https://pubmed.ncbi.nlm.nih.gov/27510637/ - DOI - PubMed
-
- Barber TM, Joharatnam J, Franks S. Pathogenesis and management of adiposity and insulin resistance in polycystic ovary syndrome (PCOS). Contemp Endocrinol. 2017:629-642. Published online October 12, 2017. doi:10.1007/978-3-319-68192-4_36 - DOI
-
- Singh S, Pal N, Shubham S, et al. Polycystic ovary syndrome: etiology, current management, and future therapeutics. J Clin Med. 2023;12(4):1454. doi:10.3390/jcm12041454. Pubmed link: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9964744/ - DOI - PMC - PubMed
-
- Hong S, Sung Y-A, Hong YS, et al. Non-alcoholic fatty liver disease is associated with hyperandrogenism in women with polycystic ovary syndrome. Sci Rep. 2023;13(1). doi:10.1038/s41598-023-39428-4. Pubmed link: http://www.ncbi.nlm.nih.gov/pmc/articles/pmc10435477/ - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

