Pegloticase and Methotrexate Cotherapy in Patients With Uncontrolled Gout With Prior Pegloticase Monotherapy Failure: Findings of an Open-Label Trial
- PMID: 39846239
- PMCID: PMC11755117
- DOI: 10.1002/acr2.11789
Pegloticase and Methotrexate Cotherapy in Patients With Uncontrolled Gout With Prior Pegloticase Monotherapy Failure: Findings of an Open-Label Trial
Abstract
Objective: Patients with uncontrolled gout have few treatment options. Pegloticase lowers serum urate (SU) levels, but antidrug antibodies limit SU-lowering response and increase infusion reaction (IR) risk. Methotrexate (MTX) cotherapy increases pegloticase response rates and lowers IR risk in pegloticase-naïve patients. Therefore, the question of re-treating patients with previous pegloticase monotherapy failure has arisen. The ADVANCE open-label trial examined pegloticase plus MTX cotherapy efficacy and safety following pegloticase monotherapy failure.
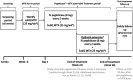
Methods: Patients with uncontrolled gout (SU levels ≥6 mg/dL, oral urate-lowering therapy failure or intolerance, and ≥1 gout sign or symptom) who previously lost SU-lowering response to pegloticase monotherapy were included. Key exclusion criteria were moderate-to-severe IR or anaphylaxis to pegloticase, MTX contraindication, immunosuppressant administration, glucose-6-phosphate dehydrogenase deficiency, and estimated glomerular filtration rate <30 mL/min/1.73m2. After a 6-week subcutaneous MTX run-in (at 25 mg/wk), patients entered 24-week pegloticase (at 8 mg biweekly) plus MTX treatment. The primary end point was SU-lowering response rate during month 6 (SU levels <6 mg/dL for ≥80% of weeks 20-24). Safety was assessed via adverse events (AEs) and laboratory monitoring.
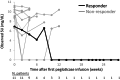
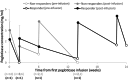
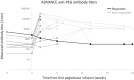
Results: Eleven patients began pegloticase plus MTX treatment (91% male patients, mean age 58.6 ± 11.3 years, mean ± SD SU levels 8.5 ± 3.2 mg/dL, 91% tophaceous). Previous pegloticase course was 2 to 27 infusions, with the last infusion admins being a mean ± SD of 3.7 ± 2.4 years before. One patient (9%) maintained response during month 6; 10 patients prematurely discontinued treatment (loss of SU lowering [n = 8], IR [n = 2]). Eight patients (73%) experienced ≥1 AE, most commonly gout flare. All AEs were mild or moderate.
Conclusion: Pegloticase plus MTX response rate following failed monotherapy was lower (9% vs 71%) and IR rate was higher (18% vs 4%) than in pegloticase-naïve patients. These findings demonstrate the challenge of overcoming established antipegloticase antibodies and emphasize the importance of initiating immunomodulation before the first pegloticase exposure.
© 2025 The Author(s). ACR Open Rheumatology published by Wiley Periodicals LLC on behalf of American College of Rheumatology.
Figures

References
-
- Sundy JS, Baraf HS, Yood RA, et al. Efficacy and tolerability of pegloticase for the treatment of chronic gout in patients refractory to conventional treatment: two randomized controlled trials. JAMA 2011;306(7):711–720. - PubMed
-
- Keenan RT, Botson JK, Masri KR, et al. The effect of immunomodulators on the efficacy and tolerability of pegloticase: a systematic review. Semin Arthritis Rheum 2021;51(2):347–352. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

