Endothelial FOXM1 and Dab2 promote diabetic wound healing
- PMID: 39846251
- PMCID: PMC11790024
- DOI: 10.1172/jci.insight.186504
Endothelial FOXM1 and Dab2 promote diabetic wound healing
Abstract
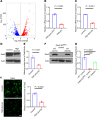
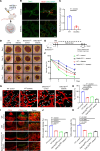
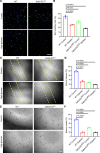
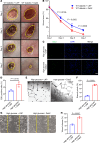
Diabetes mellitus can cause impaired and delayed wound healing, leading to lower extremity amputations; however, the mechanisms underlying the regulation of vascular endothelial growth factor-dependent (VEGF-dependent) angiogenesis remain unclear. In our study, the molecular underpinnings of endothelial dysfunction in diabetes are investigated, focusing on the roles of disabled-2 (Dab2) and Forkhead box M1 (FOXM1) in VEGF receptor 2 (VEGFR2) signaling and endothelial cell function. Bulk RNA-sequencing analysis identified significant downregulation of Dab2 in high-glucose-treated primary mouse skin endothelial cells. In diabetic mice with endothelial deficiency of Dab2, in vivo and in vitro angiogenesis and wound healing were reduced when compared with wild-type diabetic mice. Restoration of Dab2 expression by injected mRNA-containing, LyP-1-conjugated lipid nanoparticles rescued impaired angiogenesis and wound healing in diabetic mice. Furthermore, FOXM1 was downregulated in skin endothelial cells under high-glucose conditions as determined by RNA-sequencing analysis. FOXM1 was found to bind to the Dab2 promoter, regulating its expression and influencing VEGFR2 signaling. The FOXM1 inhibitor FDI-6 reduced Dab2 expression and phosphorylation of VEGFR2. Our study provides evidence of the crucial roles of Dab2 and FOXM1 in diabetic endothelial dysfunction and establishes targeted delivery as a promising treatment for diabetic vascular complications.
Keywords: Adaptor proteins; Angiogenesis.
Figures

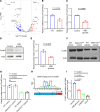
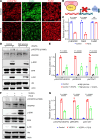
Update of
-
Interplay Between FoxM1 and Dab2 Promotes Endothelial Cell Responses in Diabetic Wound Healing.bioRxiv [Preprint]. 2024 Aug 27:2024.02.07.579019. doi: 10.1101/2024.02.07.579019. bioRxiv. 2024. Update in: JCI Insight. 2025 Jan 23;10(2):e186504. doi: 10.1172/jci.insight.186504. PMID: 39253510 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

